The Application of Nanomaterials for the Rescue of a Single Compromised Tooth with a Multidisciplinary Approach: Case Report and Scoping Review
Abstract
Background
A nanomaterial is defined as an insoluble or biopersistent and intentionally manufactured material with one or more external dimensions, or an internal structure on the scale from 1 nm to 100 nm. They are commonly considered as those materials in which the shape and molecular composition at a nanometer scale can be controlled. This extension of nanotechnology in the field of dentistry is termed “Nanodentistry”, and it has expanded to every single branch of dentistry, such as restorative, endodontic, prosthetic, and periodontology.
Case Report
The principal aim of the study was to report a noteworthy case of a 35-year-old male whose central incisor with Class III Heithersay EICR (external invasive cervical resorption) was managed through a multidisciplinary approach and the employment of various nanomaterials. Furthermore, a narrative review was also performed to investigate the state of the art of nanomaterials in different fields of modern dentistry, analyzing their application and characteristics for the recovery of a single compromised tooth. The primary sources were selected through the use of search engines, such as Pubmed (Medline), EBSCO, and Cochrane Library.
Results
Using the MeSH and non-MeSH terms and applying the search strategy previously described, a total of 442 articles were selected through search engines, such as Pubmed (Medline), EBSCO, and Cochrane Library. Titles and abstracts were screened and then full texts of all potentially relevant publications were obtained and reviewed independently. Through the application of inclusion and exclusion criteria, a total of 20 articles were selected.
Conclusion
The present case report, as well as the review of the literature, emphasize that nowadays, the adhesive systems available allow a minimally invasive treatment, which also ensures excellent aesthetic and functional results avoiding the loss of a tooth with a high aesthetic value. Interactions between different biomaterials and nanoparticles (bioceramics, sealers, nanocomposites, radicular dentin, and adhesive cementation) and correct tissue response have been reported. Further studies are needed on the topic.
1. INTRODUCTION
A nanomaterial is defined as “an insoluble or biopersistent and intentionally manufactured material with one or more external dimensions, or an internal structure, on the scale from 1 nm to 100 nm” [1]. Nanomaterials are commonly considered as those materials in which the shape and molecular composition at a nanometer scale can be controlled [1-7]. They can be classified as metal nanoparticles, non-metal ceramic nanoparticles, semiconductor nanoparticles, and carbon nanoparticles, which is well-known type [2]. Three main fields of application are diagnosis, drug administration, and regenerative medicine [1, 3]. In the field of dentistry, nanomaterials may be involved in teeth-whitening, polishing pastes for the enamel surface, dental implant coatings, dental filling, anti-sensitivity agents, and the prevention of caries [1].
Nanotechnology has allowed significant improvements in medicine and healthcare due to the ability to mimic the nanostructure of the tooth surface and nanosized organic components and the inherent properties of nanomaterials [3].
This extension of nanotechnology in the field of dentistry is termed “Nanodentistry”, and it is revolutionizing every aspect of dentistry. It consists of therapeutic and diagnostic tools and supportive aids to maintain oral hygiene with the help of nanomaterials [4]. Nanodentistry has expanded to every single branch of dentistry, such as restorative, endodontic, prosthetic, and periodontology.
The nanosize of the materials allows them to exhibit properties not present in their larger-in-scale counterparts; the high versatility of the use of nanomaterials makes them a powerful tool in dental clinics. Some features belonging to nanocomposites (nanoparticles) are as follows: more strength, durability compared to composites, flexibility, smaller dimensions with larger size ratio, improved mechanical properties, high functionality, increased wear resistance and hardness, decreased thermal expansion, and polymerization shrinkage [5].
Some nanoparticles are used for oral diseases, as preventive drugs, prostheses, and tooth implants. Nanomaterials further deliver oral fluids or drugs, preventing and curing some oral diseases (oral cancer) and maintaining oral health care up to a high extent [6].
Several research efforts have been conducted, especially during the last decade, for the improvement of the properties of materials used in dentistry; it has also been shown how these materials are improving the treatments in mainly all important areas of dentistry.
In the presented clinical case, a multidisciplinary approach was employed, involving the use of countless nanomaterials proper to different dental branches in order to achieve the rescue of a compromised tooth. The narrative review carried out in support of the case report was aimed to reveal which materials currently are more indicated and recommended for tooth conservative rehabilitation and their application according to the severity of clinical dental conditions.
2. CASE STUDY
2.1. Search Strategy
The principal aim of the review was to investigate the application of nanomaterials in different fields of modern dentistry, especially for the rescue of a single compromised tooth.
The narrative review was conducted between 1st July 2022 and 31st August 2022. The primary sources were selected through the use of search engines, such as Pubmed (Medline), EBSCO, and Cochrane Library. Alternative sources, such as Opengray literature, Google Scholar, and bibliographic indexes of previous systematic reviews related to the topic, have been consulted. The last research for a partial record update was performed on 10th October 2022. No time restriction was applied during articles’ selection. The Boolean operators used were “AND” and “OR”.
Prior to the process of selecting scientific articles for carrying out the narrative review, the inclusion and exclusion criteria were determined. Included in the study were bibliographic reviews, systematic reviews, meta-analyses, randomized controlled trials, cohort studies, case reports, and studies in English, Italian, Spanish, and Portuguese. The exclusion criteria were as follows: articles not related to the topic, animal studies, full-text not available, and articles in other languages.
Through the application of keywords and MeSH terms (such as “Nanoparticles”, “Nanostructure”, and “Dentistry”) based on a search strategy, the query string formulated is as follows: (Nanomaterials) OR (Nanoparticles) OR (Nanostructures) AND (Dentistry) AND (Conservative) OR (Restorative) OR (Composite) OR (Microhybrid) OR (Bonding) OR (Radicular Dentine) AND (Endodontic) OR (Bioceramic) OR (Fiber Post) AND (Prosthodontic) OR (Dental Crown) OR (Marginal Seal) AND (Tissue Response).
In the research phase of the scientific articles, no time restrictions were applied regarding the dates of publication of the sources.
Three operators (F.A.V, A.K, and E.L) independently managed the research and screening of the sources, applying the previously described inclusion and exclusion criteria. The results were compared and extracted, and in case of discrepancies, a senior author (R.A) was consulted in order to find a fair and thoughtful compromise. The data were collected by compiling tables of results. No meta-analysis or statistical investigations were conducted for this study.
Table 1.
| Search Query On Pubmed |
(“nanostructures”[MeSH Terms] OR “nanostructures”[All Fields] OR “nanomaterial”[All Fields] OR “nanomaterials”[All Fields] OR (“nanoparticle s”[All Fields] OR “nanoparticles”[MeSH Terms] OR “nanoparticles”[All Fields] OR “nanoparticle”[All Fields]) OR (“nanostructural”[All Fields] OR “nanostructuration”[All Fields] OR “nanostructure s”[All Fields] OR “nanostructured”[All Fields] OR “nanostructures”[MeSH Terms] OR “nanostructures”[All Fields] OR “nanostructure”[All Fields] OR “nanostructuring”[All Fields] OR “nanostructurization”[All Fields])) AND (“dentistry”[MeSH Terms] OR “dentistry”[All Fields] OR “dentistry s”[All Fields]) AND (“conservancies”[All Fields] OR “conservancy”[All Fields] OR “conservancy s”[All Fields] OR “conservation”[All Fields] OR “conservational”[All Fields] OR “conservations”[All Fields] OR “conservative”[All Fields] OR “conservatively”[All Fields] OR “conservatives”[All Fields] OR “conserve”[All Fields] OR “conserved”[All Fields] OR “conserves”[All Fields] OR “conserving”[All Fields])) OR (“restorability”[All Fields] OR “restorable”[All Fields] OR “restorated”[All Fields] OR “restoration”[All Fields] OR “restoration s”[All Fields] OR “restorations”[All Fields] OR “restorative”[All Fields] OR “restoratives”[All Fields] OR “restore”[All Fields] OR “restored”[All Fields] OR “restores”[All Fields] OR “restoring”[All Fields]) OR (“composite”[All Fields] OR “composite s”[All Fields] OR “composited”[All Fields] OR “composites”[All Fields] OR “compositing”[All Fields] OR “composition”[All Fields] OR “compositional”[All Fields] OR “compositions”[All Fields]) OR (“microhybrid”[All Fields] OR “microhybrids”[All Fields]) OR (“bonded”[All Fields] OR “bondings”[All Fields] OR “bonds”[All Fields] OR “object attachment”[MeSH Terms] OR (“object”[All Fields] AND “attachment”[All Fields]) OR “object attachment”[All Fields] OR “bonding”[All Fields]) OR (“Radicular”[All Fields] AND (“dentin”[MeSH Terms] OR “dentin”[All Fields] OR “dentine”[All Fields] OR “dentines”[All Fields] OR “dentins”[All Fields] OR “dentin s”[All Fields] OR “dentinal”[All Fields] OR “dentine s”[All Fields]))) AND (“endodontal”[All Fields] OR “endodontic”[All Fields] OR “endodontical”[All Fields] OR “endodontically”[All Fields] OR “endodontics”[MeSH Terms] OR “endodontics”[All Fields])) OR (“bioceramic”[All Fields] OR “bioceramics”[All Fields]) OR ((“dietary fiber”[MeSH Terms] OR (“dietary”[All Fields] AND “fiber”[All Fields]) OR “dietary fiber”[All Fields] OR “fiber”[All Fields] OR “fibre”[All Fields] OR “fiber s”[All Fields] OR “fiberized”[All Fields] OR “fibers”[All Fields] OR “fibre s”[All Fields] OR “fibres”[All Fields]) AND “Post”[All Fields])) AND (“prosthodontically”[All Fields] OR “prosthodontics”[MeSH Terms] OR “prosthodontics”[All Fields] OR “prosthodontic”[All Fields])) OR (“crowns”[MeSH Terms] OR “crowns”[All Fields] OR (“dental”[All Fields] AND “crown”[All Fields]) OR “dental crown”[All Fields]) OR ((“margin s”[All Fields] OR “marginal”[All Fields] OR “marginals”[All Fields] OR “margined”[All Fields] OR “margins of excision”[MeSH Terms] OR (“margins”[All Fields] AND “excision”[All Fields]) OR “margins of excision”[All Fields] OR “margin”[All Fields] OR “margins”[All Fields]) AND (“seals, earless”[MeSH Terms] OR (“seals”[All Fields] AND “earless”[All Fields]) OR “earless seals”[All Fields] OR “seal”[All Fields]))) AND ((“tissue s”[All Fields] OR “tissues”[MeSH Terms] OR “tissues”[All Fields] OR “tissue”[All Fields]) AND (“response”[All Fields] OR “responses”[All Fields] OR “responsive”[All Fields] OR “responsiveness”[All Fields] OR “responsivenesses”[All Fields] OR “responsives”[All Fields] OR “responsivities”[All Fields] OR “responsivity”[All Fields])) |
|---|
2.2. Focused Questions of the Narrative Review
The analysis of nanomaterials and their application in daily dentistry led the authors to introduce some questions, which are as follows:
- Which bonding system is recommended when the radicular and intraradicular dentin is involved? [8-12]
- Nanofillers and microhybrids: which one should be used? [13-22]
- Which endodontic cement guarantees greater results in terms of apical healing and seal? [23-32]
- Which clinical aspects should be taken into consideration for fiber posts? [33-38]
- Which indirect prosthetic restoration guarantees a good seal and satisfactory esthetics? [39-60]
- What is the response of periodontal tissues to the conservative therapy implemented? [61-82]
The narrative review has been used to respond to all questions formulated. The main goal was to show the different applications of nanomaterials in daily clinical practice. Different fields of dentistry, such as conservative, endodontic, prosthodontic, and periodontology, have been considered.
3. RESULTS
Using the MeSH and non-MeSH terms and applying the search strategy previously described, a total of 442 articles were selected through search engines, such as Pubmed (Medline), EBSCO, and Cochrane Library. Utilizing Pubmed search, 368 articles were obtained. The search through the Cochrane Library led to a total of 66 articles, while the search through the EBSCO led to a total of 8 articles. Titles and abstracts were screened, and then full texts of all potentially relevant publications were obtained and reviewed independently. Through the application of inclusion and exclusion criteria, a total of 20 articles were selected.
4. DISCUSSION
4.1. Introduction of the Case Report
The patient was a 35-year-old male who reported to the Istituto Stomatologico Italiano (ISI) in Milan (Italy). He referred to a light pain on the sextant II region and his apprehension about the appearance of pinkish discoloration in the cervical margin of tooth #21. When his medical history was taken, the patient stated that he was not affected by any chronic disease and was in good health (ASA 1). A detailed anamnesis did not report a history of trauma or other relevant issues.
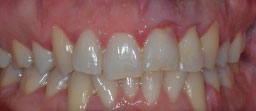
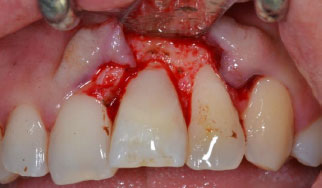
A suspicion that an EICR lesion was present was raised immediately by conducting the clinical oral examination; he exhibited general gingival inflammation, but mainly a distinctive gum defect in tooth #21 (Fig. 1). Bleeding on probing was present, and on the other hand, the percussion and pulp sensitivity thermal test provided a normal result.
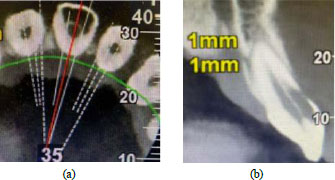
In order to confirm the suspicion of EICR, a radiographic examination was also realised; a periapical radiograph was first performed, but in this case, the realisation of a CBCT imaging was essential to assess the stage of the lesion and which structures are compromised (Fig. 2a, b). Considering the severity of the defect, it was classified as a Heithersay class III, and the treatment plan included a surgery phase involving the restoration of the defect, the endodontic treatment, and finally, the rehabilitation of the element with a lithium disilicate crown.
4.2. Focused Questions
4.2.1. Which Bonding System is Recommended when the Radicular and Intraradicular Dentin is Involved?
Dentin is a vital, permeable, elastic, and avascular tissue. Despite adhesion to dental enamel being a technique with great instant micromechanical retention established between conditioned enamel and adhesive monomers, adhesion to dentin is still a major clinical challenge to tackle [8]. There are important differences between coronal and root dentin. These differences are correlated with the number, density, and diameter of the dentinal tubules, which decrease in the apical direction and the amount of intertubular dentin [8, 9].
Adhesive systems interact with the dentin tissue following two different procedures: they can remove the smear layer (etch-and-rinse technique) or maintain it as the substrate for the bonding procedure (self-etch technique); the self-etch system is characterized by the application of an etchant/primer solution that is only air-dried (no rinsing after etching). The acidic compound remains entrapped within the modified smear layer, and the acid is buffered by the mineral released from the substrate [21]. Some studies have shown that when thick smear layers are prepared, the bond strength values of self-etching adhesive systems decrease [21]. Etch-rinse systems are the most commonly used adhesive systems for the treatment of root dentin because, in addition to promoting a more effective removal of the smear layer, a more uniform demineralization pattern is obtained [8]. Optimum root canal cleaning, adequate postselection, and absolute isolation for effective humidity control are necessary clinical approaches to guarantee long-term durable adhesive results [8]. Some authors suggest that bond strength to radicular dentin might be maximized by adopting procedures that compensate for polymerization stresses [10]. Elsaka et al. [11] showed the RealSeal self-etching primer incorporating 0.03%-0.25% (W/W) chitosan solution, a specific nanoparticle, to exhibit remarkable 7-day antibacterial activity against E. faecalis compared to the unmodified primer, and incorporation of chitosan into RealSeal self-etching primer did not affect significantly the push-out bond strength of the RealSeal system to radicular dentin. Zhou et al. [12] demonstrated that the incorporation of 1.0% chlorhexidine into the ED primer of Panavia F could extend the bond longevity of fiber post to radicular dentin and it had no negative effect on the immediate bond strength. Breschi et al. [21] reported that, within the root canal, the use of one-step self-etch adhesives is not recommended with chemical/dual composite, while the 2-step etch-and-rinse adhesives should be employed only in association with the chemical activator in order to avoid the adverse acid-base reaction; the use of light curing still remains mandatory to obtain a complete adhesive polymerization. Three-step etch-and-rinse and two-step self-etch adhesives did not show incompatibility with chemical/ dual-cured resin composites due to the use of an intermediate bonding resin layer, which is less acidic and less hydrophilic resin and prevents the negative acid-base reaction since the composite layer does not come into direct contact with the acidic monomer components in the primer layer and, thus, could reduce the permeability of the resin–tooth interfaces [21, 22].
While self-etch adhesive systems are characterized by simultaneous demineralization and infiltration, impregnation of etch-and-rinse monomers occurs after demineralization. For this reason, etch-and-rinse systems should be applied on wet dentin to maintain large interfibrillar spaces between the demineralized collagen fibrils, facilitating monomer impregnation [21].
Pleffken et al. demonstrated that the active application of self-etching adhesive systems tends to increase the dentin shear bond strength and guarantees both micromechanical and chemical bonding [69].
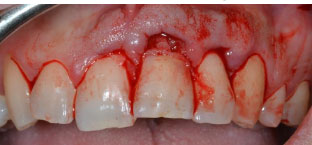
In the case reported, the first step was a surgery session; after administering local anaesthesia (articaine 4% with epinephrine 1:100.000, Pierrel Capua, Italy), a sulcular incision was made, and a full-thickness flap was carried out (Fig. 3). Once the defect was exposed, the resorptive granulation tissue was accurately removed and the site was debrided and cleaned with surgical straight handpieces (Ultimate Power+, B.A. International, Italy) and with a tungsten carbide round ball bur. Rubber dam isolation was placed (Fig. 4a) with a 212 clamp (Ivory®, Kulzer Nordic AB, Helsingborg Sweden) fixed on tooth #21. Then, the remaining tissue affected by the lesion was eliminated, which led to the exposure of the root canal; a guttapercha cone was inserted into the canal in order to restore the defect without obliterating the endodontic space with the composite (Fig. 4b). The common steps for a composite restoration described were followed: the cavity was prepared by etching the enamel and dentin with orthophosphoric acid 37% and applying a bonding system (3M™ Scotchbond™ Universal, Minnesota, USA). The defect was filled and restored with flowable composite (FiltekTM Universal Restorative, 3M Company, Minnesota, USA) (Fig. 4c), and lastly, the restoration was accurately finished and polished (Fig. 5). Finally, the flap was repositioned with an interrupted 4\0 PTFE suture, ice application was recommended, and a twice-daily oral rinse with 0,20% chlorhexidine was prescribed for 7 days following the surgery.
4.2.2. Nanofillers and Microhybrids: Which one should be used?
Microhybrid resin composites increase filler loading (more than 60% in volume) with reduced size (mean particle size from 0.4 to 1.0 μm). Nanofilled resin composites contain significantly smaller filler particles than those in conventional hybrid resin composites [13, 15].
Nanocomposites contain a unique combination of two types of nanofillers (5–75nm) and nanoclusters. Nanoparticles are discrete non-agglomerated and non-aggregated particles being 20–75nm in size. Nanocluster fillers are loosely bound agglomerates of nanosized particles. The agglomerates act as a single unit enabling high filler loading and high strength [13]. The main advantage is the reduction of the polymerization shrinkage and the increase in the mechanical properties, such as tensile strength and compressive strength to fracture. These properties of nanocomposites seem to be equivalent or sometimes even higher than hybrid composites [13]. Rastelli et al. [14] reported nanofilled composite resins to show mechanical properties at least as good as those of universal hybrids, and they could thus be used for the same clinical indications as well as for anterior restorations due to their high aesthetic properties.
Sadeghi et al. [15] showed, in their study, that in an 18-month observation period, micro-hybrid, packable, and nanofilled resin composite restorations placed in class I cavities in molar teeth exhibited minor changes compared to the baseline, and there were no significant differences between the three materials tested. Similar results were reported by Tuncer et al. [16], who demonstrated in a 5-years study that none of the microhybrid (Filtek Z250) and nanofilled (Filtek Supreme XT) composites failed; the success rate was 100% for both composite materials. Microhybrid and nanofilled composites can result in high-quality restorations, and produce positive long-term outcomes in occlusal cavities. de Andrade et al. [17] provided evidence that the materials performed satisfactorily over the 12-month observation period, but all composites under investigation, such as nanocomposites, microhybrids, and nanohybrids, showed a certain amount of deterioration related to marginal quality over time in class I cavities.
Barve et al. [18] reported that the microhardness decreased for both materials, nanofillers and microhybrids, after immersion in all beverages, such as tea, coffee, and cola. Percentage change in microhardness was considerably higher in nanocomposites in comparison to the microhybrids in cola drinks. The color change was significantly higher in nanocomposites in water and coffee. Cola drinks caused the maximum change in microhardness among all beverages and coffee caused the maximum color change among all beverages [18]. Daud et al. [19] described that the nano- and microhybrid composite surface could be affected in a simulated toothbrush, leading to an increase in surface irregularity.
Palanappian et al. [20] demonstrated that the mean vertical and volume wear of the nanofilled group was not significantly different from the microhybrid group at the four- and five-year recall. The volume wear rate of the two restorative materials was significantly influenced by factors, such as operator and cavity type.
As described in the previous section, the use of a nanofiller resin composite was preferred in this case report. The FiltekTM Universal (3M Company, Minnesota, USA) was the choice, a composite that provides optimal mechanical properties and aesthetic characteristics, permitting it to be used in anterior restorations.
4.2.3. Which Endodontic Cement Guarantees Greater Results in Terms of Apical Healing and Seal?
Root canal sealers applied during endodontic treatment have the ability to fill irregularities present between the core material and the canal wall, and infiltrate deep into the dentinal tubules; most of them possess some antimicrobial properties that sometimes decrease after setting [5, 21-23]. Nano- particles of silver (NAg) have strong antibacterial properties in low concentrations. Baras et al. [23] developed an endodontic sealer that contained 5% of dimethyl- aminohexadecyl methacrylate and 0,15% of NAg yielded in a flow; the authors demonstrated that the incorporation of these two particles did not negatively affect the film thickness and sealing properties and greatly reduced polysaccharide production by the biofilms. A similar study was conducted by Afkhami et al. [24]; the authors reported that the incorporation of silver nanoparticles into the AH Plus sealer could not improve its bacterial leakage resistance properties. The main microorganism associated with the failure of endodontic treatment is Enterococcus faecalis; Loyola-Rodriguez et al. [25] tested the application of some nanoparticles, such as chitosan (CsNPs) and Nag, in endodontic sealers in order to determine their antimicrobial effectiveness. The results showed that CsNPs have a wide spectrum of activity and a high killing rate against Gram-positive bacteria, especially when combined with chlorhexidine [25]. Vilela Teixera et al. [26] analyzed the incorporation of the nanostructured silver vanadate decorated with silver nanoparticles (AgVO3 at 2.5%, 5%, and 10%) into three endodontic sealers, and as a result of their study, the authors could not affirm that the AgVO3 promoted differences in the antibacterial activity of fresh sealers against Enterococcus faecalis. The same author, two years later, conducted a study to evaluate the cytotoxic and genotoxic effects of commercial endodontic sealers of different compositions incorporated with AgVO3 and the ionic release [31]. The authors reported that the reduction in cell viability of human gingival fibroblasts was caused by the composition of the commercial endodontic sealers and the incorporation of AgVO3; however, cell death by necrosis or apoptosis was not observed [31].
Nanoparticles of chlorhexidine have recently been incorporated into various materials, like silicone-based inputs, glass ionomer sealers, biomedical materials, and implant surfaces [25, 27]. Carvallho et al. [27] demonstrated that the introduction of nanoparticles did not compromise the radiopacity and pH of endodontic sealers; MTA Fillapex, if compared to AH plus and Pulp Canal Sealer, offered the most expressive results after the incorporation of chlorhexidine-hexametaphosphate nanoparticles. Basically, its antimicrobial properties derive from its dissociation and release of calcium and hydroxyl ions, promoting alkalinization. The AH Plus led to no expressive results after the incorporation of these particles because of the resin matrix of the sealer, which is less soluble [27]. Similar results were obtained in an in vitro study conducted by Shakya et al. [29], who analyzed the antimicrobial propriety of endodontic sealers without the incorporation of nanoparticles. The authors reported that the highest microbial inhibition was shown by calcium hydroxide cement, followed by MTA Fillapex, AH Plus, and GuttaFlow 2.
Silver nanoparticles could also be used against Staphyl- ococcus aureus, a facultative anaerobic gram-positive coccus, which has been recovered from several oral sites. As described by Parvekar et al. [28], silver nanoparticles also generate reactive oxygen species and free radicals, which damage the bacterial cell wall and inhibit the respiratory enzymes; moreover, they disturb the DNA replication and terminate the bacteria. Another nanoparticle that showed great results in terms of antibacterial and antimycotic proprieties was the multiwalled carbon tube, as described by Marica et al. [30]. Commercial sealers, with the incorporation of carbon tubes, chlorhexidine, and silver particles, showed excellent results towards E. faecalis, which is responsible for the primary etiologic factors in pulp and periapical lesions and perfect interfacial adaptation with the root canal dentine [30, 31].
Bioceramic-based root canal sealer, like BioRootTM RCS, is one of the recent root canal sealers based on tricalcium silicate material that has adhesive properties to the root canal walls and bioactive properties that may induce hard tissue deposition. Carbon nanotubes, titanium carbide, and boron nitride could significantly improve the properties of ceramic materials and their structural strength [32]. Baghdadi et al. reported that adding nanomaterials, such as carbon nanotubes and titanium carbide, to BioRootTM RCS significantly improved its compressive strength without affecting its main composition, while boron nitride had no effect on its microstructure and compressive strength. So, these nanomaterials provided higher compressive strength and significantly prevented the spread and release of microcracks [32].
The future of dentistry, especially endodontics, will change with nanotechnology, which has an immense potential of improving healthcare and human life [5].
In the case reported, ten days after surgery, the sutures have been removed and the orthograde root canal treatment was carried out in the same session. Even though the tooth was not necrotic and vitality tests were positive, root canal treatment and canal therapy became necessary because the perforating lesion involved the root canal. Cleaning and shaping of the canals were carried out by rotary file (Proteper gold, Dentsply Maillefer, Ballaigues, Switzerland), 5% NaOCl irrigation (NICLOR 5, OGNA, Muggiò, Milano), and 17% EDTA (OGNA, Muggiò, Milano).
After drying the canal with paper points, the root canal was filled with a #40 master cone and premixed bioceramic sealant (Fill Root, ST Dental World Srl, Bari) using the single-cone technique. Once the canal was definitely filled, the post space was realized, and subsequently, a fiber post was inserted. A postoperative X-ray was also realized.
4.2.4. Which Clinical Aspects should be Taken into Consideration for Fiber Post?
The restoration of an endodontically treated fractured tooth has remained a challenge for restorative dentistry for decades. Fiber posts along with composite resin core material have become more frequent in restoring endodontically treated compromised teeth because of properties, like modulus of elasticity resembling dentin, high retention, better translucency, and better transmission of forces with reinforcement of restoration and esthetics [33]. Gbadebo et al. [34] reported the clinical performance of the glass fiber post to be slightly better than that of the metallic post within the 6 months study period in their randomized clinical trial (RCT). Similar results were described by Schmitter et al. [35], wherein a sample of one hundred patients, the survival rate of fiber post was 93,5%, while for metal post, it was 75,6%. Moreover, metal posts were associated with unfavorable complications, such as root fractures. Some authors analyzed the possible interaction between the bond strength of cemented fiber posts and nanoparticles, for instance, silver nanoparticles and silver diamine fluoride (SDF), which were used as antibacterial pretreatment irrigants [36, 37].
The effect of pretreatment with silver antibacterial agents prior to adhesive cementation of fiber posts depends on the resin cement used. Silver nanoparticles had beneficial or no significant effect on bonding with cements, such as etch-and-rise cement (Variolink N), self-etch cement (Panavia F2.0), and self-adhesive cement (Panavia Luting Plus). Contrary, SDF exhibited a deleterious effect on self-adhesive cement (Panavia Luting Plus) [36].
The most common failure associated with fiber posts is their debonding at this interface resulting from problems with dentin hybridization that can be affected by irrigants, root regions, post-space preparation, and adhesive systems. Sodium hypochlorite (NaOCl), for example, had no significant effect on bond strength and interface permeability. As reported by Shafiei [36], Suzuki et al. [37] demonstrated that silver nanoparticles dispersion had no negative effects on the adhesion and interface permeability values between the glass fiber posts and the intraradicular dentin. The authors reported in detail that the decreased hydrophilicity of the RelyX U200 resin cement, compared to the Maxcem Elite resin, seemed to result in more favorable bonding results. The Maxcem Elite resin cements were more prone to water absorption. The increased water permeability accelerated the hydrolytic degradation of the cement [37].
Following the results of authors that have been previously described, Jowkar et al. [38] reported that the intraradicular dentin pretreatment with silver nanoparticles, zinc oxide nanoparticles, and titanium oxide nanoparticles did not interfere with the push-out bond strength of fiber post-to-root dentin using Variolink II and Panavia F2.0.
Root canal therapy and fiber post placement was performed in the same appointment in the present case review; a 3M fiber post was placed and 3MTM RelyXTM ultimate adhesive resin cement was used for cementation.
4.2.5. Which Indirect Prosthetic Restoration Guarantees a Good seal and Satisfactory Esthetics?
This is the question that every clinician should ask when faced with the restoration of an extremely aesthetically dental element, as the one presented in this case report.
Prosthetic materials in dentistry have undergone a major evolution over the years, and many changes have been made in order to improve mechanical, physical, and biological properties. Traditional metal-ceramic restorations used to be considered the best option as reliable materials. However, the increasing aesthetic demand has promoted the introduction of metal-free restorations, commonly called ceramic materials [39]. Conventionally, ceramic materials could be categorized into glass ceramics, alumina-based ceramics, and zirconia-based ceramics, based on their chemical composition [40]. Glass matrix ceramics have always been appreciated and employed in dentistry, considering their aesthetic properties (biomimetic appearance and high translucency), but also for their biocompatibility characteristics given the high amount of silica in their composition [41].
Nowadays, lithium disilicate (LD) is one of the most popular restorative materials; it is a glass ceramic whose mechanical properties have been improved while preserving its natural appearance [42]. The flexural strength of more than 300 MPa ensures that LD ceramics are suitable for veneers, inlays, and onlays, but especially for single crowns; in fact, for the latter, we have the largest number of clinical studies [43-45].
In essence, there are two methods for manufacturing restoration in LD: CAD-CAM (computer-aided design and computer-aided manufacturing) and traditional heat pressing. It is widely shown that there is no significant difference in mechanical properties or aesthetic performance between the two manufacturing methods [46-48].
Accuracy of fit is the parameter most strongly related to the longevity of a restoration; countless studies have evaluated if relevant variations exist in marginal and internal adaptation depending on production methods; controversial results have been recorded, but in all cases, they have been considered clinically acceptable [49, 50].
In addition, regarding the survival rate, no statically significant differences have been recorded; however, both manufacturing methods have been deemed as reliable treatment options [45].
LD crowns could be veneered with feldspathic porcelain or used as a monolithic restoration. In the present case, monolithic prostheses were preferred, omitting the veneering process and associated complications [51]. Not surprisingly, one of the most frequent complications reported was the fracture of the ceramic veneer.
Regarding materials, the most widely used material is lithium disilicate, both for monolithic and veneered crowns, which is the IPS e.max press or CAD (Ivoclar Vivadent AG).
The cementation process of the ceramo-metal crown and also of zirconia and alumina-based ceramics is based on mechanical retention; for these reasons, zinc phosphate or glass ionomer cement are usually used. On the other hand, the high amount of silica in the glassy matrix of the LD ceramics, apart from providing them with optical quality, allows chemical adhesion with resin cement [52, 53].
Regarding composite cement, the majority of current knowledge on the topic is based on in vitro studies that have shown adhesive resin cementation, increased retention, and improved fracture resistance of lithium disilicate crowns [54, 55]. With regards to adhesive cementation, a wide range of cements have been reported in various studies, including 3-step adhesive resin cement [44, 56, 56], self-etching resin cement [57, 58], or self-adhesive resin cement [43, 58]. The factors that most influence the choice of adhesive cement type are the thickness of the restoration, the position of the restoration in the arch (anterior or posterior), and the degree of translucency. Although, it must be considered that differences in the surface morphology of ceramics could also influence the adhesion. In an in vitro study, it was shown that under the same unconditioned glass-ceramic surface, the microfracture bond strength of RelyXTM Unicem (3M Espe AG, Seefeld, Germany) was significantly higher than that of Multilink (Ivoclar Vivadent, Schaan, Liechtenstein) and Panavia F (Kuraray Medical Inc., Tokyo, Japan). However, after HF etching and ceramic silanization, statistically superior microfracture resistance values were obtained for RelyXTM, Unicem, and Multilink, compared to Panavia F [59]. However, resin or conventional cementation of full-coverage lithium disilicate restorations have been a controversial topic; a recent systematic review has concluded that adhesive and conventional cementation would produce comparable clinical results for zirconia and lithium disilicate single crowns [60]. In Maroulakos’ systematic review, the most reported complications with adhesively cemented lithium disilicate crowns were fracture of the restoration and loss of retention, while in the traditional cemented crown, only the restoration fracture was reported [60]. However, of the 2436 restorations included in the article, 1957 (80.3%) were adhesively cemented lithium disilicate crowns. If more long-term data are considered, a wider variety of complications may appear. Certainly, further well-designed randomized clinical trials on the subject are needed, but despite this, it is evident that nowadays, adhesive cementation of lithium disilicate crown remains the most commonly used and clinically valued.
For the case presented, the prosthetic phase followed gingival tissue condition assessment and periapical radiography of the tooth (Fig. 6a-b); in Fig. (6), the absence of inflammation and the healing of the gingival tissues can be seen.
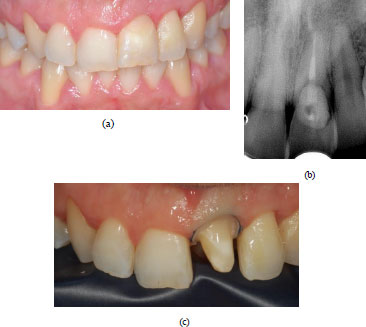
After administering local anaesthesia (articaine 4% with epinephrine 1:100.000, Pierrel Capua, Italy), the preparation of the tooth was performed; a rounded shoulder margin was chosen (Fig. 6c), which is preferred for the ceramic crown, and finally, the provisional was placed.
In this case report, as mentioned earlier, CAD-CAM manufacturing was preferred (IPS e.max CAD- Ivoclar Vivadent AG), considering the satisfactory aesthetic appearance and avoiding the complications described earlier.
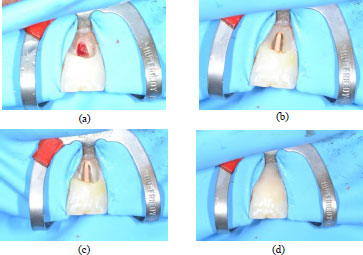
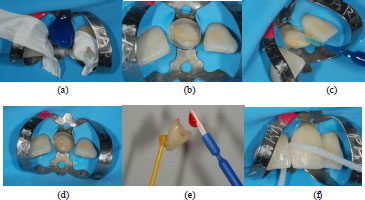
Approximately 14 days after the handing over of the provisional, the final crown was received and resin cementation steps were followed; first of all, the rubber dam isolation was placed with 9 clamps on tooth 21 and its preparation margin was exposed. Adjacent teeth were protected from etching with polytetrafluoroethylene (PTFE) tape and total acid etching was performed for 20 seconds (Fig. 7a). Then, the preparation was rinsed and lightly air-dried and a two-step bonding system was applied and light-cured for 20 seconds (Fig. 7b-d).
Next, the internal surface of the LD crown was prepared; hydrofluoric acid etching was performed for seconds and the dissolution of the glassy phase could create an appropriate microstructure for bonding. This was followed by the application of silane primer, which was carefully air-dried (Fig. 7e). The use of this agent was found to be crucial as it could form chemical bonds between the inorganic phase of the ceramic and the organic phase of the resin. Lastly, a resin cement was applied to the margins of the crown and the crown was seated. Excess cement on the crown margins was cured for 2 seconds and removed with the explorer tip, and then complete polymerization was achieved (Fig. 7f). Once it was verified that no excess cement was present, the rubber dam was removed and occlusal adjustment was checked.
4.2.6. What is the Response of Periodontal Tissues to the Conservative Therapy Implemented?
Biocompatibility is an imperative requirement for dental materials. In pursuit of this goal, several biomaterials have been intensively studied and introduced, having a positive, or at least non-damaging, impact on periodontal oral tissues. It is desirable to implement a material that avoids biofilm formation, and this is critical for the long-term success of dental crowns, which are in close contact with oral tissues for prolonged periods [61]. After all, even the aesthetic success of a crown, such as the one implemented in this case report, is closely associated with its integration with the surrounding periodontal tissue. Investigations regarding the quality of dental ceramic materials have dominated the research in the last years, but the majority of the available evidence is based on in vitro studies. In Brackett MG et al.’s investigation, they concluded that lithium disilicates and ceramics, in general, should not be considered inert biologically; although they reported LD materials to also be less cytotoxic than several commonly used composite materials and comparable to the cytotoxicity reported for several alloys and glass ionomers [62]. Indeed, in Forster's investigation on the rate of attachment and proliferation of cultured human epithelial cells, lithium disilicate (IPS e.max Press, Ivoclar Vivadent AG, Germany) exhibited the greatest biocompatibility [63]. It is a well-known fact today that zirconia and lithium disilicate ceramics have no potential toxic or genotoxic effects and they are able to achieve better in vivo integration than other materials and satisfactory soft tissue responses [64]. Besides the material used for crown manufacture, the impact on periodontal tissue is also determined by the position of the crown margin, the marginal fit, and its contour. The presence of resin cement at the level and\or below the gingival margin is a critical area because of the potential for biofilm accumulation, the possible direct irritation of gingival tissues, and the possible invasion of biological width [65]. Therefore, it is necessary to carry out the adhesion steps carefully and thoroughly check that all excess cement is properly removed. In the present case review, the composite resin was also used for the restoration of the EICR defect and as a transitional reconstruction of the cervical marginal of the element. Several materials could be used for the restoration of the EICR defect; investigations regarding the quality of mineral trioxide aggregate “MTA” (Dentsply Tulsa Dental, Johnson City, TN, USA) have dominated the research in the last years [66]. Even though the efficacy and biocompatibility of materials, such as Biodentine [67] and MTA [68], have been widely demonstrated, composite resin is preferred in this treatment. Nowadays, modern composite resins could offer an aesthetic restoration and also an adequate adhesive bond strength in ensuring in-root dentine by employing a proper self-etch adhesive system [69]. In addition, the composite resin has a fast-setting time (20 sec light-curing time) compared to the long setting time of the MTA, and the polishing of the restoration provides a smooth surface that prevents the development of subgingival plaque.
Countless studies have aimed to evaluate the biocompatibility of composite materials with periodontal tissues. First of all, the cytotoxicity of composites on cell cultures, epithelial cultures, and on connective tissue cultures has been examined in several in vitro investigations [70, 71]. In addition, positive results have been reported in histological studies conducted on animals where great biocompatibility of composite materials has been registered [72]; nevertheless, there exist many differences in experimental animal models compared to human mouth tissues and physiology [73]. Several in vivo studies have also been performed, but contradictory clinical results have been presented [74, 75]. Bertoldi et al. demonstrated that subgingival restorations, if well-shaped and well-finished, exhibit similar periodontal tissue health as natural root surfaces [76]. Furthermore, several authors, in their papers, have reported the same positive outcomes [77-85]. Human clinical studies have shown negative outcomes where inflammation and bleeding may be related to biological amplitude invasion [79, 82-85].
According to this, in the present case report, it was possible to appreciate the periodontal tissue status after the composite restoration and after the placement of the lithium disilicate crown. The findings of multiple investigations have confirmed a better behavior of lithium disilicate compared to resin composite [80-82]. Nevertheless, it is possible to appreciate the repair of the initial gingival defect that has occurred only with the composite restoration before the prosthetic element preparation (Fig. 6a). The absence of gingival inflammation and a probing depth of less than 3mm was registered at the 2-year follow-up visit, thus confirming the treatment's success and the successful integration of the gingival tissues. The complete resolution of the symptoms and the absence of a periodontal pocket also support the clinical assessment of a healthy new epithelial tissue gingival attachment. Even though composite resins and lithium disilicate are not fully biologically acceptable to periodontal tissues, the “creeping attachment” described by Goldman develops, and coronal migration of the gingival marginal tissue occurs [83-85].
4.3. Resolution of the Case Report
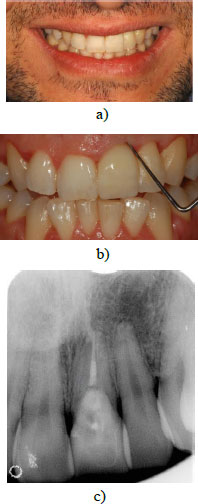
The patient was examined after 3 months and the periodontal probing revealed a healthy site without bleeding and the probing depth was less than 3mm. The patient subsequently received monitoring visits, respectively, after 3 months, 6 months, and 12 months; on each visit, a periapical radiograph was taken and a clinical examination with probing was realized. The absence of gingival inflammation and a probing depth of less than 3mm were also registered at the 2-year follow-up visit (Fig. 8a-c), thus confirming the treatment's success and the successful integration of the gingival tissues.
CONCLUSION
In the case of coronal dentine, etch-rinse systems promote an effective removal of the smear layer and a uniform demineralization, while a self-etching adhesive system tends to increase the dentin shear bond strength and guarantee both micromechanical and chemical bonding. Nanofilled composite resins have shown mechanical properties at least as good as those of universal hybrids. No drastic differences have been reported.
Nanoparticles have been reported to possess antimicrobial properties and, in association with bioceramic sealers, provide higher compressive strength and significantly prevent the spread and release of microcracks. Silver nanoparticles have beneficial or no significant effect on bonding for fiber post cementation.
Lithium disilicate, on the other hand, offers adequate mechanical properties and guarantees satisfactory esthetics. Thus, the periodontal tissue response, firstly to composite resins and secondly to the disilicate crown, would seem satisfactory. The findings of multiple investigations have confirmed a better behavior of lithium disilicate compared to resin composite.
AUTHORS’ CONTRIBUTION
The authors contributed equally to conceptualization, methodology, validation, investigation, writing, revision, editing, and supervision. All authors have read and agreed to the publication of the manuscript.


