All published articles of this journal are available on ScienceDirect.
The Potential Role of Indoleamine 2,3 Dioxygenase in the Pathogenesis of Type 2 Diabetes and Periodontitis
Abstract
Introduction
Periodontal medicine is a subdiscipline of periodontology, where the relationship between periodontal diseases and overall health is examined. Inflammation serves as a pivotal mechanism linking diabetes and periodontal disease. Indoleamine 2,3-dioxygenase (IDO) is the rate-limiting enzyme in the catabolic pathway of tryptophan, resulting in the generation of kynurenines, which regulate immune functions. Recently, IDO as a potential biomarker has been investigated in many systemic conditions with underlying inflammatory pathology. This review aimed to formulate a potential hypothesis suggesting the role of IDO as one of the common links for the bidirectional relationship between type 2 diabetes and periodontitis.
Methods
Electronic databases, including PubMed, Scopus, ProQuest, and Google Scholar, were searched using keywords, such as “indoleamine 2,3 dioxygenase,” “tryptophan-kynurenine pathway,” “diabetes,” and “periodontitis.” All study types performed in humans and animal models were included.
Results
A summary of the available literature is provided to illustrate the topics related to the potential mechanism linking the inflammatory basis of diabetes and periodontitis. The studies exploring the mechanistic pathways related to IDO in diabetes and periodontitis are also described.
Discussion
The pathways through which IDO may potentiate the link between diabetes and periodontitis are discussed based on available literature evidence. The need for maintenance of immune homeostasis through regulatory T cells and inflammatory cytokines is emphasized.
Conclusion
This literature review summarizes the mechanisms through which IDO enzyme exerts complex and multifaceted roles in immune regulation, inflammation, and tryptophan metabolism, thereby influencing the pathogenesis of periodontitis and type 2 diabetes.
1. INTRODUCTION
Periodontal medicine is a subdiscipline of periodontology that examines the relationship between periodontal diseases and overall health. Research on how various systemic conditions influence the development of periodontal disease is also performed within this discipline. Current evidence reveals that periodontal disease can affect several systemic conditions, including coronary artery disease, diabetes, preterm labor, and respiratory diseases. Among these, diabetes mellitus has been extensively studied because it shares a bidirectional relationship with periodontal disease. Patients with diabetes, particularly those with poor metabolic control, commonly exhibit an increased prevalence and severity of periodontitis compared with those of non-diabetic individuals [1]. However, periodontal disease is now regarded as a comorbidity of both conditions rather than a complication. The term “comorbidity” was coined in 1970 by epidemiologist A.R. Feinstein to describe the simultaneous existence of conditions that are not primarily dependent on one another [2]. Research on comorbidities has primarily focused on the pathobiological processes of diseases at the molecular level.
Inflammation serves as a pivotal mechanism linking diabetes and periodontal disease. Indoleamine 2,3-dioxygenase (IDO) is the initial rate-limiting enzyme in the catabolic pathway of the essential amino acid tryptophan. IDO initiates tryptophan breakdown, resulting in the generation of kynurenines, which are known to possess critical immune regulatory functions. This literature review aimed to provide the biological plausibility of IDO, which plays a significant role in the bidirectional association between diabetes and periodontal diseases.
2. METHODS
2.1. Search Strategy
PubMed, Scopus, ProQuest, and Google Scholar were searched using terms, such as “indoleamine 2,3 dioxygenase,” “tryptophan-kynurenine pathway,” “diabetes,” and “periodontitis.” A search strategy was first developed in PubMed using MeSH terms and was then adopted for other databases.
2.2. Selection Criteria
All study types performed on humans and animal models were included. No restrictions were placed on the date range or language of publication. Initial article selection was based on the title and abstract, with the inclusion of the most relevant and informative data and results. Subsequently, full texts of potentially relevant articles were retrieved and evaluated based on the predefined inclusion criteria. Furthermore, the reference lists of selected studies were manually searched to identify additional pertinent articles.
3. RESULTS
3.1. Periodontitis and Diabetes as Shared Risk Elements
Periodontal disease is a chronic inflammatory condition caused by bacterial infection that affects both the gingiva and supporting bone of the teeth. The disease spectrum ranges from a localized condition with minimal alterations to significant periodontal destruction, resulting in attachment loss and alveolar bone loss. Besides periodontopathogens, genetic and environmental factors contribute to an individual's susceptibility to chronic inflammation.
The presence of pathogenic bacteria alone is insufficient to cause periodontitis. Instead, disruption of the host’s immune-inflammatory response is crucial for disease progression. This disruption can arise from multiple factors, including the presence of periodontopathic bacteria, elevated levels of pro-inflammatory cytokines, matrix metalloproteinases, and reactive oxygen species, as well as impaired neutrophil function [3, 4].
Diabetes mellitus, a chronic metabolic disorder characterized by hyperglycemia, is a well-established risk factor for periodontal disease. Several studies have shown that individuals with diabetes, particularly those with poor glycemic control, are more susceptible to developing periodontal disease and tend to experience more severe forms compared with those experienced by their non-diabetic counterparts. The relationship between diabetes and periodontal disease is complex and bidirectional.
3.2. Inflammation and Microbial Dysbiosis as Shared Risk Elements
The interplay between periodontal inflammation and microbial imbalance is a complex phenomenon that impacts both oral and overall health. This interaction is characterized by the “Inflammation-Mediated Polymicrobial-Emergence and Dysbiotic-Exacerbation” model [5].
Periodontal inflammation, triggered by the host response to a diverse bacterial community, creates an environment that promotes dysbiosis. The resulting periodontal pockets provide a unique niche with altered oxygen levels and nutrient availability, favoring the proliferation of anaerobic and protein-degrading bacteria. This shift in microbial composition, characterized by increased species diversity, amplifies inflammation, perpetuating a cycle of tissue destruction [5].
Diabetes significantly exacerbates the inflammatory response to periodontal infections [3, 6]. Individuals with diabetes frequently exhibit impaired immune functions, including reduced neutrophil activity and altered cytokine profiles [7]. This heightened inflammatory state contributes to severe periodontal damage and accelerates disease progression in patients with diabetes [7]. Reportedly, sugar control can enhance periodontal outcomes, underscoring the impact of diabetes on periodontal health [7].
A bidirectional relationship exists between diabetes and periodontitis. While diabetes increases the risk and severity of periodontitis, periodontal inflammation can adversely affect blood sugar control and potentially exacerbate diabetes [7]. This reciprocal relationship underscores the importance of simultaneously managing both conditions for optimal patient outcomes.
Periodontal inflammation is not merely a localized oral condition; it also contributes to systemic inflammation and elevates the risk of various systemic conditions, including cardiovascular diseases and kidney complications [7, 8]. In individuals with diabetes, periodontitis increases the risk of kidney-related complications and cardiovascular mortality [6]. This underscores the significant systemic health implications of periodontal inflammation, particularly in the context of diabetes.
Type 2 diabetes (T2D) and periodontitis are chronic inflammatory diseases in which the inflammatory response plays a crucial role in their pathogenesis and interconnection. The balance among T helper (Th) 1, Th2, Th17, and T Regulatory (Treg) cells is crucial for regulating inflammatory responses in both conditions.
Effective management of this complex interplay necessitates addressing both inflammation and dysbiosis. Therapeutic approaches should aim to reduce inflammatory mediators, restore host immune function, and modulate the subgingival microbiome to disrupt the vicious cycle linking diabetes and periodontal disease [1, 3, 7, 9].
3.3. Current Understanding of T Helper Cell Response in Diabetes and Periodontitis
Both diabetes and periodontitis are chronic inflammatory diseases in which T helper cells play crucial roles in the inflammatory processes. Dysregulation of these responses contributes significantly to disease pathogenesis.
3.3.1. Th17 and Treg Cells in Periodontitis
More recently, attention has shifted towards the roles of Th17 and regulatory T cells in periodontitis. Th17 cells, which produce interleukin (IL)-17, are potent inducers of inflammation and bone resorption, contributing significantly to periodontal tissue damage [10, 11]. In contrast, Tregs play a crucial role in suppressing immune responses and maintaining homeostasis [11]. Their dysfunction or reduction in number, as noted in previous studies [11, 12], can exacerbate inflammation and disease progression in periodontitis. Additionally, Zhao et al. [10], illustrated the detrimental role of Th17 cells and the protective effect of Th2 cells in periodontitis, further emphasizing the complex interplay between these cell types.
3.3.2. Th17 and Treg Cells in Diabetes
Similar to periodontitis, Th17 and Treg cells also play pivotal roles in diabetes. Studies have shown that increased IL-17 levels and Th17 activity in type 1 diabetes (T1D) contribute to the autoimmune destruction of pancreatic beta cells [13]. Conversely, Treg cells are essential for maintaining self-tolerance and preventing autoimmunity, and their dysfunction contributes to T1D development [13].
3.3.3. Interplay between Diabetes and Periodontitis
The shared involvement of Th17 and Treg cells underscores their interconnectedness in diabetes and periodontitis. Chronic inflammation, a characteristic of both diseases, can contribute to the progression of each disease. Elevated levels of pro-inflammatory cytokines, such as IL-1β, seen in diabetes, have been observed in individuals with both diabetes and periodontitis [14]. This amplified inflammatory state further fuels the dysregulation of Th cell responses in periodontitis.
3.4. Inflammatory Basis for Complications Related to Diabetes Mellitus
Chronic inflammation is a major driver in the development and progression of complications in diabetes mellitus. Hyperglycemia induces many complications; however, the resulting inflammatory cascade mediates much of the tissue damage [15]. This inflammation occurs both locally and systemically, contributing to a range of microvascular and macrovascular complications [16].
3.4.1. Mechanisms of Inflammation in Diabetes
3.4.1.1. Hyperglycemia-Induced Inflammation
Elevated glucose levels activate multiple pathways that promote inflammation, including increased production of reactive oxygen species, activation of protein kinase C, and formation of advanced glycation end products (AGEs) [16]. The binding of AGEs to their receptors on various cell types further amplifies this inflammatory response [17].
3.4.1.2. Insulin Resistance and Inflammation
Insulin resistance, a hallmark of T2D, is closely associated with inflammation. Pro-inflammatory cytokines, such as tumor necrosis factor (TNF)-α and IL-6, interfere with insulin signaling, creating a vicious cycle in which insulin resistance promotes inflammation and vice versa [18].
3.4.1.3. Immune Cell Dysfunction
Diabetes can impair the function of immune cells, including neutrophils and macrophages, contributing to an exaggerated inflammatory response and impaired ability to clear infections [19].
3.4.1.4. Adipokines and Inflammation
Adipose tissue secretes various adipokines, including leptin and resistin, which have pro-inflammatory effects [18]. This contributes to the chronic low-grade inflammation observed in obesity and diabetes.
3.4.2. Inflammation and Diabetic Complications
Inflammation has been linked to multiple diabetic complications, including the following:
3.4.2.1. Microvascular Complications
Inflammation contributes to retinal vascular, glomerular, and nerve damage.
3.4.2.2. Macrovascular Complications
Inflammation plays a key role in atherosclerosis, stroke, and peripheral artery disease.
3.4.2.3. Infections
In individuals with diabetes, impaired immune function and reduced blood flow increase susceptibility to infections [19], ranging from skin infections to severe life-threatening conditions [16].
3.4.2.4. Other Complications
Inflammation may contribute to diabetic foot ulcers, delayed wound healing, and other medical conditions, such as middle ear infections and hearing loss [20].
3.5. Indoleamine 2,3 Dioxygenase: A Novel Connecting Link in the Inflammatory Basis of Disease
IDO is an enzyme that plays complex and multifaceted roles in immune regulation, inflammation, and tryptophan metabolism. It catalyzes the breakdown of tryptophan, an essential amino acid, into kynurenine. This pathway has several downstream effects, including the modulation of immune responses, and influences various physiological processes.
In immune regulation, IDO is primarily recognized for its immunosuppressive properties. It suppresses T cell responses, promotes immune tolerance, and facilitates immune evasion in certain conditions, such as cancer and pregnancy [21]. This immune modulation occurs partly through tryptophan depletion and the generation of kynurenine and its metabolites, which directly affect the immune cells [21].
IDO plays a crucial role in regulating the balance between Th17 and Treg cells, which are key players in the immune response. Its enzymatic activity degrades tryptophan into kynurenine, which exerts significant downstream effects on immune regulation. IDO-mediated tryptophan catabolism activates Tregs, inhibits Th17 cell differentiation, and blocks the conversion of Tregs into Th17-like cells [22, 23].
Thus, IDO promotes a shift in the Th17/Treg balance towards a more immunosuppressive state by enhancing Treg activity while inhibiting Th17 differentiation and conversion. This intricate regulation by IDO is essential for maintaining immune homeostasis and preventing excessive inflammation that can cause tissue damage and autoimmune disorders [24-26]. Dysregulation of the Th17/Treg balance has been implicated in various conditions, including T1D, inflammatory bone diseases, chronic human immunodeficiency virus infection, and childhood allergic asthma [13, 24-26].
IDO is commonly associated with immunosuppression; however, it also contributes to inflammation under certain conditions. Its activity can result in the production of pro-inflammatory kynurenine metabolites, which can exacerbate inflammation in certain diseases [27].
3.6. Indoleamine 2,3 Dioxygenase in Diabetes
Immunological processes linked to hyperglycemia-induced inflammatory response may offer important pathogenic clues and serve as diagnostic tools to assess the development and progression of diabetes and its complications. Given its critical role in modulating inflammatory responses in various pathological conditions characterized by immune dysregulation, IDO is likely to play a role in diabetes-related immunity and inflammatory processes as well.
3.6.1. Indoleamine 2,3 Dioxygenase and Type 2 Diabetes
The published literature has predominantly focused on in vitro and animal studies. The role of IDO1 in the mechanistic pathways contributing to diabetes mellitus is presented in Table 1 [28-33].
A summary of the studies in Table 1 suggests that enhanced IDO activity may represent a potential risk factor for T2D. However, it is also evident that patients with insulin resistance and T2D exhibit significantly elevated circulating IL-6 levels. This increased IL-6 production is closely linked to the development and progression of diabetic complications. As described earlier, IL-6 suppresses IDO function by promoting suppressor of cytokine signaling 3-mediated proteasomal degradation. However, the altered expression and function of IL-6 in diabetes may affect various cell types differently, including T cells, dendritic cells (DCs), and endothelial cells across different stages of disease development and progression.
| Authors | Main Findings |
|---|---|
| Oxenkrug (2010) [28] | - Up-regulated interferon-gamma (IFN-γ) production initiates an inflammation cascade involving the tryptophan-kynurenine and guanine-tetrahydrobiopterin pathways, contributing to aging and associated disorders. - The IFN-γ-induced cascade influences nitric oxide synthase activity and increases the production of kynurenine derivatives linked to diabetes, anxiety, psychoses, and cognitive impairment. - Inhibiting these pathways may slow the aging processes and provide novel therapeutic approaches for aging-associated medical and psychiatric disorders. |
| Oxenkrug (2011) [29] | - Chronic inflammation and the transcriptional induction of indoleamine 2,3-dioxygenase (IDO) by pro-inflammatory cytokines are key mechanisms in metabolic syndrome development and age-associated neuroendocrine disorders. - Genetic predisposition, through high producer alleles of cytokine genes, may result in elevated pro-inflammatory cytokine production and 'superinduction' of IDO. - The tryptophan-kynurenine metabolism is a potential target for the prevention and treatment of metabolic syndrome and age-associated neuroendocrine disorders. |
| Li et al. [30] | - Wild-type mice fed a high-fat safflower oil diet develop hepatic insulin resistance, whereas Gpat1 knockout mice remain insulin sensitive. - Metabolomics analysis identified specific metabolite alterations associated with insulin resistance, including elevated urea cycle intermediates and decreased 1,5-anhydroglucitol. - In this study, previously unrecognized metabolites linked to insulin resistance were identified, highlighting the utility of metabolomics in understanding diabetes pathophysiology. |
| Oxenkrug (2015) [31] | - Patients with type 2 diabetes (T2D) exhibited increased plasma levels of kynurenine (KYN) and its downstream metabolites, kynurenic and xanthurenic acids, compared with those in non-diabetic controls. - The findings support the "kynurenine hypothesis," which proposes that these metabolites play a role in the progression from insulin resistance to T2D. - Dysregulation of the kynurenine pathway, potentially driven by chronic stress or inflammation, may contribute to the T2D development. - Moreover, neopterin levels measured in this study were used to differentiate the role of IDO and tryptophan 2,3-dioxygenase enzymes in tryptophan metabolism, since human neopterin levels specifically reflect IFN-γ activity. Plasma neopterin levels were positively correlated with Homeostatic Model Assessment – Insulin Resistance values in this study. |
| Sarkar et al. [32] | - IFN-γ induces a marked increase in IDO mRNA and protein expression in human islets, suggesting a protective role against cytotoxic damage. - Activation of IDO by IFN-γ is accompanied by increased enzyme activity and kynurenine release; however, chronic exposure may lead to beta-cell attrition. - The JAK-STAT signaling pathway and chemokines are upregulated in response to IFN-γ, potentially exacerbating insulitis by recruiting lymphocytes. |
| Liu et al. [33] | -Demonstrated for the first time in normal rat pancreatic islets that a few genes involved in the Try-Kyn pathway are constitutively expressed. - It was also highlighted that the regulatory enzyme IDO1 is not constitutively expressed. - Additionally, it was highlighted that the expression of IDO1 and kynurenine 3-monooxygenase can be potently activated by pro-inflammatory cytokines, particularly IFN-γ. - It was concluded that oxidative stress has only a marginal influence on genes involved in the Try-Kyn pathway. |
| Authors | Main Findings |
|---|---|
| Zhang et al. [34] | - Indoleamine 2,3 dioxygenase (IDO) activity is elevated in type 2 diabetic nephropathy (DN) and negatively correlates with estimated glomerular filtration rate (eGFR), suggesting a relationship with chronic kidney disease (CKD) severity. - IDO activity is significantly higher in patients with type 2 DN than in healthy individuals and those with latent glomerulonephritis, but lower than in patients undergoing maintenance hemodialysis. - Preliminary evidence that IDO activity may contribute to the pathogenesis of type 2 DN, especially when eGFR is above 60 ml/min per 1.73 m², was provided in this study. |
| Cernaro et al. [35] | - Patients receiving renin-angiotensin system (RAS) inhibitors showed significantly lower kynurenine levels than those not receiving them. - In patients not receiving RAS inhibitors, kynurenine levels inversely correlated with eGFR and directly correlated with proteinuria and albuminuria. - Kynurenine is emerging as a potential new biomarker for CKD. |
| Hu et al. [36] | - Increased expression of IDO and quinolinic acid (QUIN) in diabetic retinas is associated with neuronal degeneration characteristic of diabetic retinopathy. - Microglia activation, along with increased IDO and QUIN expression, is linked to neuron loss in the retinas of patients with type 1 or 2 diabetes. - In diabetic retinas, local inflammation is marked by increased densities of activated microglia and macrophages. |
| Daissormont et al. [37] | - Plasmacytoid dendritic cells (PDCs) play a protective role in atherosclerosis by reducing T-cell proliferation and activity. - Depletion of PDCs increases atherosclerosis development and progression in mice. - Conversely, the ability of IDO to modulate dendritic cell activity-whether suppressive or stimulatory-has gained interest in cardiovascular medicine. The protective role of PDCs in atherosclerosis is being investigated, potentially through IDO expression, which contributes to dampening T-cell proliferation. |
3.6.2. Indoleamine 2,3 Dioxygenase and Diabetic Complications
Studies investigating the role of IDO1 in diabetic complications, including nephropathy, chronic kidney disease (CKD), and atherosclerosis, are presented in Table 2 [34-37].
A summary of the findings of the studies in Table 2 indicates that elevated IDO1 activity is associated with diabetes-related complications, including nephropathy and retinopathy. Kynurenine levels have been proposed as a potential biomarker in patients with CKD.
3.7. Indoleamine 2,3 Dioxygenase and Periodontal Disease
Periodontitis is a complex, chronic inflammatory disease in which the individual’s host response appears to influence the outcome of the disease process. Various factors, including (a) genetic and epigenetic, (b) lifestyle, (c) comorbidities, (d) local or dental, including those that act randomly, and pathobionts within a dysbiotic subgingival biofilm, play significant roles in determining an individual’s immune fitness. Loss of homeostasis may result from disruption of the host balance and an abnormal host response. This aberrant response can manifest as either hyper- or hypo-responsiveness and/or a lack of sufficient resolution of the inflammatory reactions. IDO plays a significant role in modulating the immune response, primarily through interferon-gamma.
The various mechanisms by which IDO influences the host immune response are outlined in Table 3 [38-42]. Studies have reported that IDO plays a dual role in periodontitis. Elevated IDO expression in periodontitis is attributed to its activation by inflammatory cytokines and bacterial products.
| Authors | Main Findings |
|---|---|
| Mahanonda et al. [38] | - Human gingival fibroblasts (HGFs) express mRNA for Toll-like receptors (TLRs) 1, 2, 3, 4, 5, 6, and 9, but not for TLRs 7, 8, and 10. - Stimulation with specific TLR ligands induces interleukin (IL)-8 and indoleamine 2,3 dioxygenase (IDO) expression in HGFs, with tumor necrosis factor-alpha and interferon-gamma (IFN-γ) enhancing these effects. - IDO production by HGFs inhibits T cell proliferation, suggesting a role in modulating inflammation within periodontal tissues. |
| Mahanonda et al. [39] | - IL-17 induces IL-8 production and minimal intracellular adhesin molecule 1 (ICAM-1) expression in HGFs but does not influence HLA-DR, CD40, or IDO. - IFN-γ increases HLA-DR, ICAM-1, and IDO expression but not IL-8 production. - Combined IL-17 and IFN-γ treatment significantly enhances ICAM-1, IL-8, and IDO expression, indicating a synergistic effect on immune modulation. |
| Nisapakultorn et al. [40] | - IDO expression is significantly higher in patients with periodontitis lesions than in those with healthy gingiva. - IFN-γ is a potent inducer of IDO expression and activity in human gingival fibroblasts. - The upregulation of IDO in periodontitis may result from activation by inflammatory cytokines and bacterial products. |
| Moon et al. [41] | - LPS treatment significantly increases IDO expression and activity in human periodontal ligament cells in a dose- and time-dependent manner. - Human periodontal ligament cells exhibit a stronger response to lipopolysaccharide-induced IDO expression than that in gingival fibroblasts. - Elevated kynurenine production confirms IDO functional activity in periodontal ligament cells after lipopolysaccharide treatment. |
| Qin et al. [42] | - IDO deficiency exacerbates gingival inflammation, characterized by elevated levels of proinflammatory cytokine IL-17 and reduced regulatory T cells. - The absence of IDO results in increased apoptotic and necrotic cell death in gingival tissues. - IDO is a pivotal regulator of LPS-induced gingival inflammation, suggesting its potential as a therapeutic target for managing gingivitis. |
IDO deficiency has also been associated with increased susceptibility to periodontal inflammation, along with elevated apoptotic and necrotic cell death in gingival tissues.
4. DISCUSSION
4.1. The Role of Indoleamine 2,3 Dioxygenase as a Potential Link Between Diabetes and Periodontitis
IDO's role as a potential link between diabetes and periodontitis is an area of ongoing research. While a causal link has not been definitively established, several lines of evidence suggest that IDO is involved in the complex interplay between these two diseases (Fig. 1).
4.1.1. Indoleamine 2,3 Dioxygenase and Inflammation
IDO is an enzyme playing a crucial role in immune regulation and inflammation. It catabolizes tryptophan, producing kynurenine and other metabolites. Based on the context and specific metabolites, these products can exert both immunosuppressive and immune-activating effects. This dual nature permitted us to understand the complex role of IDO in various diseases.
4.1.2. Indoleamine 2,3 Dioxygenase in Diabetes
Chronic, low-grade inflammation contributes to the progression and complications of T2D. Buckzo et al. reported altered tryptophan metabolism, including changes in the kynurenine and kynurenic acid levels, in the saliva of patients with T2D and hypertension [43]. Some studies have suggested the potential role of IDO in the pathogenesis of diabetes; however, the detailed mechanism is still being investigated [44, 45]. It is important to note that current literature mainly indicates an association between IDO and diabetes, not causation. Further studies are warranted to understand the precise role of IDO in diabetes.
4.1.3. Indoleamine 2,3 Dioxygenase in Periodontitis
Periodontitis, a chronic inflammatory disease impacting the gums and supporting teeth structures, shares multiple inflammatory pathways with diabetes mellitus. IDO is expressed in the periodontal tissues and may be involved in modulating immune responses within the gingival environment [44]. Studies have suggested that IDO influences the balance of immune cells in the gingiva, particularly regulatory T cells, which are important in controlling inflammation [44]. Some evidence suggests that IDO activity is both protective and detrimental in periodontitis [45]. Although its immunomodulatory effects can reduce inflammation and promote tissue repair, dysregulation of IDO activity may contribute to chronic inflammation and tissue destruction.
The main outcomes of studies assessing IDO and its potential mechanisms linking type 2 diabetes and periodontitis are summarized in Table 4 [43-45].
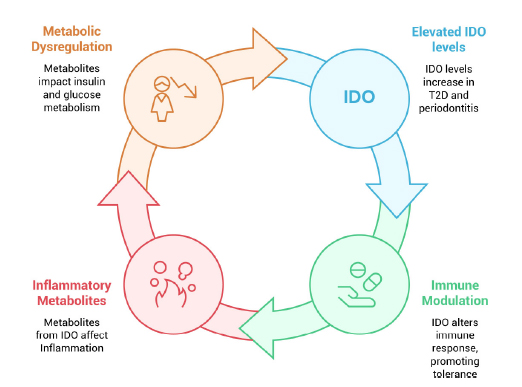
Potential role of IDO in type 2 diabetes and periodontitis.
| Authors | Main Findings |
|---|---|
| Buczko et al. [43] | - Kynurenine and kynurenic acid concentrations were significantly higher in patients with both diabetes and hypertension than in healthy volunteers and those with only one condition. - No differences were observed in the concentrations of 3-hydroxykynurenine and anthranilic acid among the studied groups. - Altered tryptophan metabolism through the kynurenine pathway may contribute to the increased severity of periodontal disease in diabetic patients with hypertension. |
| Tyagi et al. [44] | - Porphyromonas gingivalis induces monocytes to differentiate into dysfunctional dendritic cells, promoting dysregulated immune responses and chronic infection. - Pathogen-infected dendritic cells show prolonged survival in humanized mice, suggesting a mechanism for persistent infection. - Indoleamine 2,3-dioxygenase (IDO) plays a crucial role in inducing regulatory T cells, thereby promoting disease progression and pathogen immune escape. |
| Thomas et al. [45] | - IDO is a key enzyme in the kynurenine pathway, essential for tryptophan metabolism and involved in various physiological and pathophysiological processes. - Kynurenine pathway metabolites exhibit both antioxidant and pro-oxidant activities, influencing immune and inflammatory responses. - Redox reactions play a significant role in regulating IDO activity and tryptophan metabolism, impacting various biological functions. |
4.1.4. Hypothetical Link between Diabetes Mellitus and Periodontitis
Given IDO's involvement in both diabetes and periodontitis, it is reasonable to consider it a potentially common link. The chronic inflammatory state characteristics of both diseases could influence IDO’s activity and contribute to their bidirectional relationship. For example, elevated inflammatory mediator levels in diabetes may exacerbate periodontal inflammation, possibly through mechanisms involving IDO. Conversely, periodontal inflammation can contribute to systemic inflammation, thereby affecting metabolic control in diabetes. Specifically, the ability of IDO to modulate Treg activity can influence the course of inflammation during periodontitis (Fig. 2).
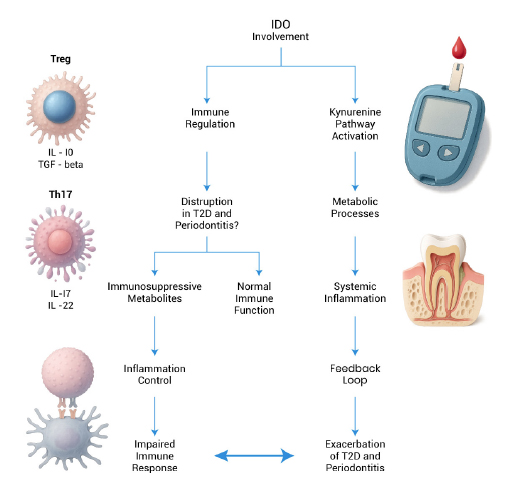
IDO’s role as a potential link between diabetes and periodontitis.
4.2. Limitations and Scope for Further Research
This is a narrative review exploring the available literature evidence on the role of IDO in type 2 diabetes and periodontitis. Further research is warranted to fully elucidate IDO's role in the relationship between diabetes and periodontitis. Investigating IDO activity in individuals with both diseases and exploring the potential of IDO modulation as a therapeutic target could provide valuable insights and treatment strategies. Identifying specific drug targets to modulate this pathway can be essential in subverting downstream complications associated with both chronic disease conditions.
SUMMARY AND CONCLUSION
In summary, the role of IDO in the interplay between T2D and periodontitis is an active area of research. IDO is involved in immune regulation and inflammation, which are central to both diseases. While a direct causal link has yet to be firmly established, current evidence suggests that IDO may serve as a potential common mechanism connecting these two complex conditions.
Ongoing research to better understand the intricacies of IDO’s involvement, along with exploring therapeutic strategies targeting IDO, may offer valuable insights and novel approaches for managing the diabetes-periodontitis interrelationship.
AUTHORS’ CONTRIBUTIONS
The authors confirm contribution to the paper as follows: R.S. and V.L.: Study conception and design, draft manuscript; R.S.: Data collection; R.S., V.L., V.V. and R.A.: Analysis and interpretation of results. All authors reviewed the results and approved the final version of the manuscript.
LIST OF ABBREVIATIONS
| IDO | = Indoleamine 2 3-dioxygenase |
| MeSH | = Medical Subject Headings |
| T2D | = Type 2 Diabetes |
| Th Cells | = T Helper Cells |
| Treg | = T Regulatory Cells |
| IL | = Interleukin |
| T1D | = Type 1 diabetes |
| AGEs | = Advanced Glycation End products |
| TNF | = Tumor Necrosis Factor |
| DCs | = Dendritic Cells |
| CKD | = Chronic Kidney Disease |
CONSENT FOR PUBLICATION
All authors have reviewed the manuscript and provided their consent for publication.
ACKNOWLEDGEMENTS
The authors would like to thank Editage (www.editage.com) for English language editing.


