All published articles of this journal are available on ScienceDirect.
Clinical and Salivary Antioxidant Effects of Matcha Tea in Patients with Gingivitis: A Randomized Controlled Trial
Abstract
Introduction
Gingivitis, the initial stage of periodontal disease, is characterised by inflammation driven by dental biofilm and associated with oxidative stress. Matcha tea, a powdered green tea rich in antioxidants, has shown potential health benefits. This study aimed to investigate the effect of Matcha tea consumption on clinical periodontal parameters and salivary antioxidant levels in patients with gingivitis.
Methods
A randomised controlled clinical trial was conducted with 41 participants diagnosed with gingivitis. Participants were randomly allocated to a Matcha tea group (n=21) who consumed two cups of Matcha tea daily for 30 days, or a control group (n=20) who received standard oral hygiene instructions. Primary outcomes were clinical periodontal parameters (Plaque Index [PI], Bleeding on Probing percentage [BOP%], Gingival Index [GI]), and secondary outcomes were salivary levels of Malondialdehyde (MDA), Superoxide Dismutase (SOD), and Glutathione Peroxidase 1 (GPX1), assessed at baseline and after 30 days.
Results
Both groups showed significant within-group improvements in PI, BOP%, and GI at the 30-day follow-up (p<0.01). However, there were no statistically significant differences in these clinical parameters between the Matcha tea and control groups at the endpoint. The Matcha tea group exhibited a significant reduction in salivary MDA levels (p=0.002) and significantly higher salivary SOD (p=0.047) and GPX1 (p=0.027) levels compared to the control group at the 30-day follow-up.
Discussion
The biochemical improvements are attributed to Matcha's potent antioxidants, which favorably modulate the oral redox balance. The lack of superior clinical outcomes suggests that the profound effect of mechanical plaque control may have overshadowed any adjunctive benefits from Matcha within the 30-day trial. This highlights a potential disconnect between systemic antioxidant modulation and localised clinical changes in gingivitis management.
Conclusion
Daily consumption of Matcha tea for one month favorably modulated salivary antioxidant biomarkers in patients with gingivitis, reducing lipid peroxidation and increasing antioxidant enzyme levels. However, these biochemical changes did not translate into statistically significant differences in clinical periodontal parameters beyond standard oral hygiene within this study's timeframe.
1. INTRODUCTION
Periodontal health is integral to overall well-being, defined not merely as the absence of disease but as a state free from inflammatory periodontal conditions, allowing normal function without adverse consequences [1, 2]. Periodontal diseases encompass a group of chronic, destructive inflammatory conditions affecting the tooth-supporting structures, representing a significant global health burden with high prevalence worldwide [3, 4]. The primary etiological factor is the accumulation of dental biofilm on tooth surfaces, initiating a complex interaction between microbial communities and the host immune system [5, 6].
Periodontal disease is broadly classified into gingivitis and periodontitis [7]. Gingivitis, the initial stage, is characterized by inflammation confined to the gingival tissues, typically presenting as redness, swelling, and bleeding on probing (BOP), without loss of periodontal attachment or alveolar bone [8-10]. It results from a dysregulated host immunoinflammatory response to a dysbiotic plaque biofilm and is considered reversible with adequate biofilm control [8, 9]. BOP is a key objective sign for diagnosing and monitoring gingivitis [11-13]. If unresolved, gingivitis can progress to periodontitis, characterized by irreversible destruction of the periodontal ligament and alveolar bone, which can ultimately lead to tooth loss [14, 15].
Oxidative stress, an imbalance between reactive oxygen species (ROS) production and antioxidant defense capacity, plays a significant role in periodontal disease pathogenesis [16, 17]. Excessive ROS production, often linked to inflammatory responses involving neutrophils, can damage periodontal tissues through lipid peroxidation, protein damage, and DNA damage [18, 19]. Malondialdehyde (MDA) serves as a well-established biomarker of lipid peroxidation and oxidative stress, with elevated levels found in saliva and associated with gingivitis and periodontitis [20-22].
The body relies on antioxidant defense systems to counteract ROS-induced damage [23, 24]. Key enzymatic antioxidants include Superoxide Dismutase (SOD), which catalyzes superoxide radical dismutation, and Glutathione Peroxidase 1 (GPX1), a selenium-containing enzyme that reduces hydrogen peroxide and organic hydroperoxides [25, 26]. Alterations in SOD and GPX1 levels have been observed in periodontal disease, reflecting ongoing oxidative challenge [27-30]. Saliva offers a convenient, non-invasive medium for assessing oxidative stress status in the oral cavity [31, 32].
Tea, derived from Camellia sinensis, is the second most consumed beverage globally and is associated with numerous health benefits, including antioxidant and anti-inflammatory properties [33-35]. Matcha, a fine-powdered green tea, is unique due to its shade-cultivation process, resulting in higher concentrations of theanine, caffeine, chlorophyll, and certain antioxidants like Vitamin C and rutin compared to other green teas [36-38]. Recent clinical studies have demonstrated that green tea interventions, including mouthwashes and systemic consumption, can reduce plaque accumulation and gingival inflammation [39-41]. Matcha possesses potent antioxidant and anti-inflammatory compounds, including catechins (like EGCG), phenolic acids, and quercetin, which may contribute to periodontal health benefits [42-44]. In vitro studies have shown that green tea polyphenols exhibit antimicrobial effects against key periodontal pathogens, including Porphyromonas gingivalis and Aggregatibacter actinomycetemcomitans [45, 46]. Systematic reviews and meta-analyses have indicated that green tea interventions can provide modest but significant improvements in periodontal parameters when used as adjuncts to conventional therapy [47, 48]. Despite growing evidence for green tea's periodontal benefits, research specifically investigating Matcha tea's effects on gingivitis and salivary oxidative stress biomarkers remains limited. Therefore, this study aimed to investigate the effect of consuming Matcha tea on salivary antioxidant levels (SOD, GPX1), a marker of lipid peroxidation (MDA), and clinical periodontal parameters (BOP, PI, GI) in patients diagnosed with dental biofilm-induced gingivitis.
2. MATERIALS AND METHODS
2.1. Study Design
This study was conducted as a randomized controlled clinical trial from December 2023 to May 2024. Participants were recruited from the patient pool at the clinics of the Periodontics Department, College of Dentistry, Kufa University, Najaf, Iraq. The trial was registered with ClinicalTrials.gov (Identifier: NCT06912958).
2.2. Ethical Considerations
The study protocol received ethical approval from the Ethics Committee of the College of Dentistry, University of Baghdad (Ref. number: 883623, dated 3/12/2023), covering the research activities conducted at the clinics of the Periodontics Department, College of Dentistry, Kufa University. All procedures performed involving human participants were conducted by the ethical standards of the institutional research committee and adhered to the principles outlined in the Declaration of Helsinki. Before enrollment, all participants received a complete description of the study's nature and aims. Written informed consent was obtained from each participant. Participants were informed of their right to withdraw from the study at any time without justification. Signed consent forms were collected and securely stored.
2.3. Participant Selection
2.3.1. Inclusion Criteria
To be eligible for participation in the study, individuals were required to be in good systemic health and possess at least 20 natural teeth. Participants needed to be non-smokers and must not have received any medical or dental treatment known to impact periodontal status within the six months prior to the examination. Clinically, eligibility necessitated a diagnosis of generalized gingivitis, defined as having Bleeding on Probing (BOP) at more than 10% of sites, Probing Pocket Depths (PPD) equal to or less than 3mm at all sites (assuming no pseudo-pockets), and the absence of any Clinical Attachment Loss (CAL). Furthermore, participants were required to have a plaque index score of 1, according to the modified Quigley-Hein index.
2.3.2. Exclusion Criteria
Individuals were excluded from the study if they were pregnant or currently breastfeeding, were current smokers, or were regular alcohol consumers. The presence of orthodontic appliances also precluded participation. Additionally, individuals with a reported history of systemic diseases, such as diabetes mellitus or cardiovascular conditions, or those diagnosed with periodontitis (characterized by the presence of CAL or PPD exceeding 3mm) were ineligible for enrollment.
2.4. Sample Size
The power analysis was conducted using G*Power version 3.1, setting standard parameters: a desired statistical power (1-β) of 80% and a significance level (α) set at 0.05 for a two-tailed test. Based on the effect size estimated from the pilot data, the analysis indicated that a minimum of 22 participants per group was required to achieve 80% power to detect the anticipated difference as statistically significant if it truly exists.
To accommodate potential participant attrition (dropouts or loss to follow-up) expected during the one-month study period, the calculated sample size was inflated by approximately 15%. Therefore, the final target recruitment was set at 26 participants per group to maintain adequate statistical power for the final analysis. Although the target was 26 per group, 22 were successfully randomized to each group at the study's commencement.
2.5. Intervention and Randomization
Participants were randomly allocated into two groups using simple randomization (coin flip):
2.5.1. Matcha Tea Group
Consumed Matcha tea (Organic Japanese Matcha, Jade Leaf Japan, Lot T47110p100).
2.5.2. Control Group
Received only standard oral hygiene instructions and motivation.
The allocation process was performed by an examiner who was not involved in assessing study variables. The primary examiner was blinded to the intervention allocation. Interventions were assigned codes (A or B) by a third party uninvolved in the study. These codes were kept in sealed envelopes and were revealed only at the end of the study during data analysis.
Participants in the Matcha tea group were instructed to consume two cups of Matcha tea per day for 30 days. They were supplied with standardized tea packages and cups and oral and written instructions on preparing the tea. Participants in the control group received the exact standardized oral hygiene instructions and motivation as the intervention group.
2.6. Outcome Measures
2.7. Examiner Calibration
To ensure reliability of clinical measurements, the examiner underwent intra-examiner calibration before commencing the study. Ten subjects (not part of the main study) were assessed for PI, GI, and BOP twice, with a two-hour interval between measurements. The calibration sessions were repeated until an acceptable level of agreement was achieved, defined as a Kappa value >0.75. The final achieved Kappa value for the clinical parameters in this study was 0.9.
2.8. Data Collection Procedures
Clinical examinations and saliva collections were performed at baseline and after 30 days (endpoint).
2.8.1. Clinical Examination
All clinical parameters were recorded for all teeth present (excluding third molars) by a single calibrated examiner.
2.8.1.1. Plaque Index (PI)
The Quigley-Hein plaque index (modified by Turesky et al.) was used [49]. Teeth surfaces were wiped, disclosed using a biofilm disclosing agent (EMS Switzerland) applied with tweezers, and gently rinsed. Plaque presence (score 1) or absence (score 0) was recorded on four surfaces per tooth (buccal, lingual, mesial, distal). The mean PI score per patient was calculated. Patients were shown disclosed plaque using mirrors to reinforce oral hygiene motivation.
2.8.1.2. Bleeding on Probing (BOP)
Recorded dichotomously (presence=1, absence=0) at six sites per tooth (mesiobuccal, mid-buccal, distobuccal, mesiolingual, mid-lingual, distolingual) using a UNC-15 periodontal probe (PHOENIX, UK). The probe was gently inserted into the sulcus until slight resistance was felt. Bleeding occurring within 30 seconds of probing was recorded as positive. The percentage of sites bleeding per patient (BOP%) was calculated.
2.8.1.3. Gingival Index (GI)
Assessed using the Löe and Silness index [50], at four gingival areas per tooth. Scores ranged from 0 to 3: 0=Normal gingiva; 1=Mild inflammation (slight color change, slight edema, no BOP); 2=Moderate inflammation (redness, edema, glazing, BOP); 3=Severe inflammation (marked redness, edema, ulceration, tendency to spontaneous bleeding). The mean GI score per patient was calculated.
Standard dental equipment was used, including dental mirrors (PHOENIX, UK), disposable tweezers, cotton rolls, and an autoclave (Melag, Germany) for sterilization.
2.8.2. Saliva Collection
Participants were instructed to refrain from eating, drinking, and performing oral hygiene procedures for at least two hours before collection. They rinsed their mouths with tap water for 30 seconds, approximately 10 minutes prior to collection. Unstimulated whole saliva (approx. 3ml) was collected by expectoration into sterile tubes while the participant was seated upright. Samples were immediately placed in a cooling box with ice packs (Kish Termoos, China). Within one hour, samples were centrifuged (Yunlinli, China) at 3000 rpm for 20 minutes. The clear supernatant was carefully aspirated using a micropipette into labeled Eppendorf (EP) tubes (1.5ml, CBWTC/OEM, Jiangsu, China) and stored at -20°C until biochemical analysis.
2.9. Biochemical Analysis (ELISA)
Salivary concentrations of MDA, SOD (Cu-Zn), and GPX1 were quantified using commercially available ELISA kits according to the manufacturer's instructions (BT LAB, Bioassay Technology Laboratory, China).
2.9.1. Assay Principle
All kits utilized a sandwich ELISA principle. Pre-coated plates with specific antibodies (anti-Human MDA, anti-Human SOD1, or anti-Human GPX1) were used. Saliva samples (or standards) were added, followed by biotinylated detection antibody and Streptavidin-HRP conjugate. After incubation and washing steps to remove unbound components, a substrate solution was added, developing color in proportion to the analyte concentration. The reaction was stopped with an acidic solution, and absorbance was measured at 450 nm.
2.9.2. Reagent Preparation
Standard solutions for each analyte were serially diluted as per the kit instructions to create a standard curve. Wash buffer concentrate (25x) was diluted with distilled water. All reagents and samples were brought to room temperature before use.
2.9.3. Assay Procedure
All ELISA procedures were performed according to the manufacturer's instructions (BT LAB, Bioassay Technology Laboratory, China) for each specific analyte (MDA, SOD, GPX1). Initially, appropriate volumes of standards and saliva samples were pipetted into the designated wells of the pre-coated microplates; the sample volume for the MDA assay was 40 µL, while volumes for the SOD and GPX1 assays adhered to their respective kit protocols. Subsequently, 10 µL of biotinylated detection antibody, followed by 50 µL of Streptavidin-HRP conjugate, was added sequentially to each well. The microplates were then covered with a plate sealer, gently mixed, and incubated for 1 hour at 37°C. Following this incubation, the plates underwent a washing procedure consisting of five cycles using the prepared wash buffer, with a 1-2 minute soak time included in each cycle. Next, substrate solution A and substrate solution B were added to each well, and the plates were incubated at 37°C in the dark for the duration specified by the manufacturer's protocol to allow for color development. The enzymatic reaction was terminated by adding 50 µL of stop solution to each well. Finally, the optical density (absorbance) of the color developed in each well was measured at 450 nm using a microplate reader within 10 minutes of adding the stop solution.
2.9.4. Calculation
Concentrations of MDA (nmol/ml), SOD (nmol/ml), and GPX1 (µU/ml) in the saliva samples were determined by interpolating their absorbance values against the respective standard curves generated using non-linear regression analysis provided by the reader software or plotted manually.
2.10. Statistical Analysis
Statistical analyses were performed using SPSS version 28.0 (IBM Corp., Armonk, NY, USA) with significance set at α = 0.05. Data normality was assessed using the Shapiro-Wilk test. Continuous variables were described as means ± standard deviations or medians with interquartile ranges. Categorical variables were presented as frequencies and percentages.
Given the non-parametric distribution of most variables, within-group comparisons used the Wilcoxon signed-rank test, and between-group comparisons used the Mann-Whitney U test. Categorical variables were analyzed using chi-square or Fisher's exact tests. Spearman's rank correlation assessed relationships between clinical parameters and salivary biomarkers at one-month follow-up across all participants (n=41).
3. RESULTS
3.1. Participant Recruitment and Baseline Characteristics
The flow of participants through this randomized controlled trial is detailed in the CONSORT diagram (Fig. 1). Following initial screening of 50 individuals, 44 met the eligibility criteria and were randomized, with 22 allocated to the Matcha tea group and 22 to the control group. Three participants (one from the Matcha group, two from the control group) were lost to follow-up during the one-month study period. Consequently, 41 participants (Matcha tea group, n=21; Control group, n=20) completed the study protocol and were included in the final analysis.
Baseline demographic characteristics, presented in Table 1, were well-balanced between the two groups. The mean age was 26.68 ± 6.515 years in the Matcha tea group and 28.86 ± 9.926 years in the control group, with no statistically significant difference. Sex distribution was also comparable between the groups. All participants entered the study with a diagnosis of gingivitis. As detailed in Table 2, the initial distribution favored localized gingivitis in both groups (70% Matcha, 52% Control), with the remainder presenting with generalized gingivitis. Notably, at the one-month follow-up, no participants in either group exhibited generalized gingivitis.
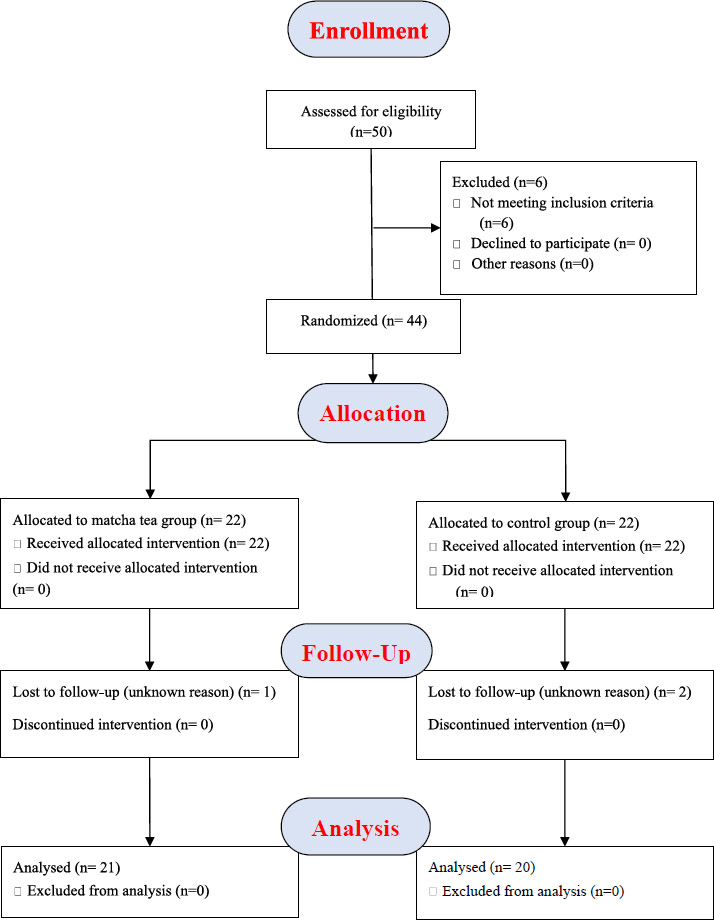
CONSORT flow chart.
| Characteristic | Matcha Group (n=21) | Control Group (n=20) | Total (n=41) | p-value |
|---|---|---|---|---|
| Age (years), Mean ± SD | 26.68 ± 6.515 | 28.86 ± 9.926 | 27 ± 8.489 | NS |
| Sex, n (%) | - | NS | ||
| Male | 8 (38.1%) | 7 (35.0%) | 15 (36.6%) | |
| Female | 13 (61.9%) | 13 (65.0%) | 26 (63.4%) | |
| Intervention Group | Baseline | One Month | ||
|---|---|---|---|---|
| Localized (%) | Generalized (%) | Localized (%) | Generalized (%) | |
| Matcha tea (n=21) | 70.0 | 30.0 | 40.0 | 0.0 |
| Control (n=20) | 52.0 | 48.0 | 28.6 | 0.0 |
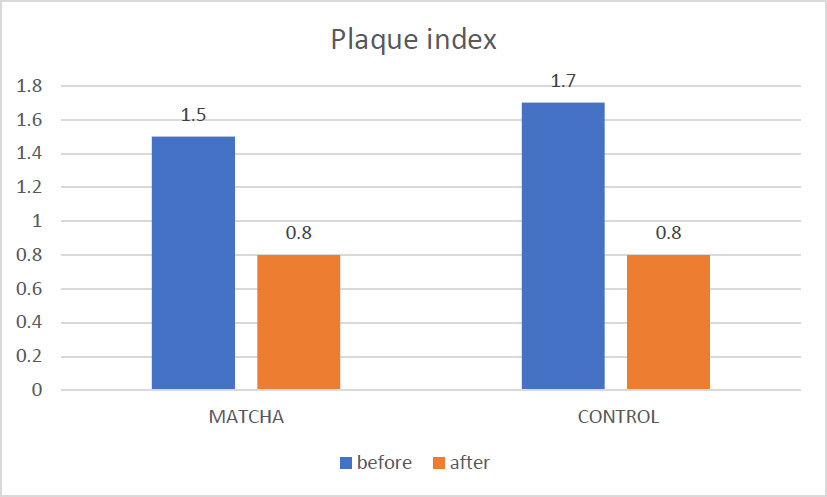
Mean PI before and after intervention.
3.2. Clinical Periodontal Outcomes
From baseline to the one-month follow-up assessment, the Matcha tea and control groups observed statistically significant improvements in all clinical periodontal parameters (Plaque Index, Bleeding on Probing percentage, Gingival Index).
3.2.1. Plaque Index (PI)
As illustrated in Fig. (2), the mean PI score decreased significantly within the Matcha tea group from 1.5 ± 0.56 at baseline to 0.8 ± 0.43 at follow-up (Wilcoxon Signed Ranks test, p < 0.001). A similar significant reduction was documented in the control group, from a baseline mean PI of 1.7 ± 0.43 to 0.8 ± 0.37 at follow-up (Wilcoxon Signed Ranks test, p < 0.01). An inter-group comparison of the mean PI scores at the one-month follow-up, detailed in Table 3, revealed no statistically significant difference between the Matcha tea and control groups (p = 0.74).
3.2.2. Bleeding on Probing Percentage (BOP%)
Fig. (3) depicts the changes in BOP percentage. A statistically significant reduction in mean BOP% was observed in the Matcha tea group, declining from 39.9 ± 13.8% at baseline to 18.0 ± 10.7% after one month (Wilcoxon Signed Ranks test, p < 0.001). The control group demonstrated a comparable significant decrease in mean BOP%, from 44.3 ± 14.1% to 21.6 ± 12.5% (Wilcoxon Signed Ranks test, p < 0.01). Analysis of the mean BOP percentage at follow-up (Table 3) indicated no statistically significant difference between the two study groups (p = 0.34).
3.2.3. Gingival Index (GI)
Significant improvements in gingival inflammation were evident in both groups, as shown in Fig. (4). The mean GI score in the Matcha tea group decreased significantly from 1.1 ± 0.63 at baseline to 0.6 ± 0.49 at the one-month assessment (Wilcoxon Signed Ranks test, p < 0.001). The control group also significantly reduced mean GI, from 1.4 ± 0.51 to 0.7 ± 0.40 (Wilcoxon Signed Ranks test, p < 0.01). The mean GI scores at the one-month follow-up were not significantly different between the Matcha tea and control groups (p = 0.38), as summarized in Table 3.
3.3. Salivary Biomarker Levels
The effects of Matcha tea consumption on salivary oxidative stress markers and antioxidant enzyme levels were evaluated.
| Parameter | Group | n | Baseline Mean ± SD | Follow-up Mean ± SD | Mean Change (Follow-up - Baseline) ± SD | Within-Group p-value† | Inter-Group p-value (Follow-up)‡ |
|---|---|---|---|---|---|---|---|
| PI | Matcha | 21 | 1.5 ± 0.56 | 0.8 ± 0.43 | -0.7 ± NA | < 0.001*** | 0.74 |
| Control | 20 | 1.7 ± 0.43 | 0.8 ± 0.37 | -0.9 ± NA | < 0.01** | ||
| GI | Matcha | 21 | 1.1 ± 0.63 | 0.6 ± 0.49 | -0.5 ± NA | < 0.001*** | 0.38 |
| Control | 20 | 1.4 ± 0.51 | 0.7 ± 0.40 | -0.7 ± NA | < 0.01** | ||
| BOP% | Matcha | 21 | 39.9 ± 13.8% | 18.0 ± 10.7% | -21.9 ± NA | < 0.001*** | 0.34 |
| Control | 20 | 44.3 ± 14.1% | 21.6 ± 12.5% | -22.7 ± NA | < 0.01** |
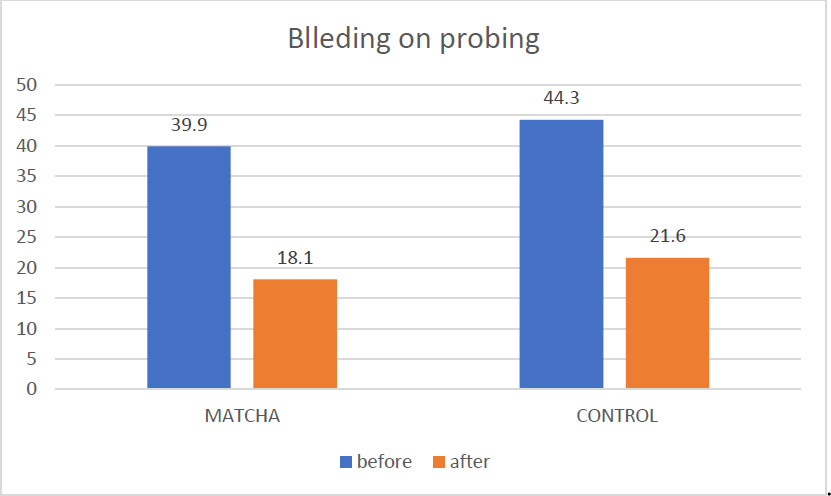
Mean BOP% before and after intervention.
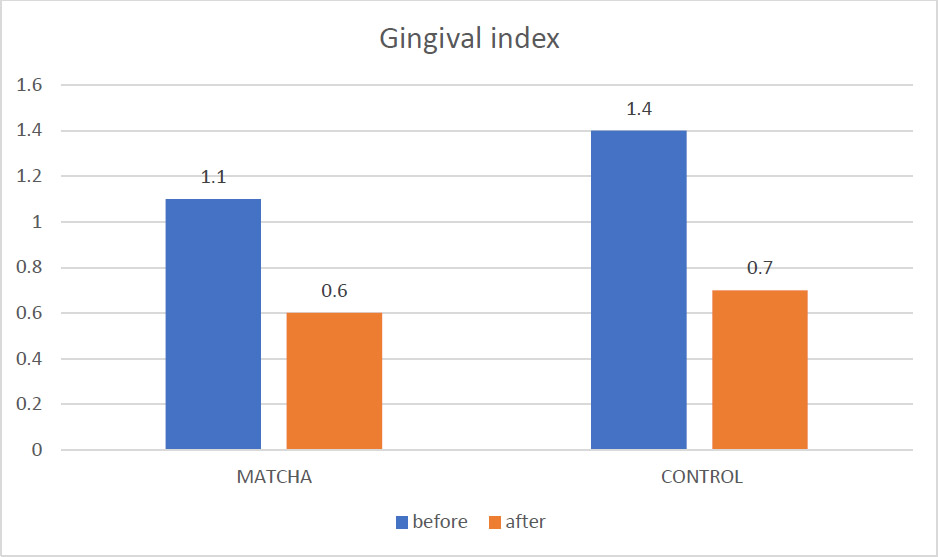
Mean GI before and after intervention.
3.3.1. Malondialdehyde (MDA)
Fig. (5) illustrates the salivary MDA levels. In Table 4, a statistically significant reduction in mean MDA concentration was observed within the Matcha tea group, from 19.5 ± 5.2 U/mL at baseline to 15.6 ± 5.9 U/mL at the one-month follow-up (Wilcoxon Signed Ranks test, p = 0.002). Conversely, no significant change in MDA levels was detected within the control group (Baseline: 13.4 ± 4.9 U/mL; Follow-up: 12.6 ± 3.4 U/mL; Wilcoxon Signed Ranks test, p = 0.476). Despite the significant reduction within the Matcha group, comparing mean MDA levels between the two groups at the one-month point did not reveal a statistically significant difference (p = 0.183).
3.3.2. Superoxide Dismutase (SOD)
As shown in Fig. (6) and Table 4, mean salivary SOD levels did not change significantly from baseline to follow-up within either the Matcha tea group (Baseline: 28.5 ± 15 U/mL; Follow-up: 31.9 ± 15.7 U/mL; p = 0.296) or the control group (Baseline: 24.8 ± 13.2 U/mL; Follow-up: 22.5 ± 9.4 U/mL; p = 0.498). However, a direct comparison of the groups at the one-month follow-up indicated that the mean salivary SOD concentration was statistically significantly higher in the Matcha tea group relative to the control group (p = 0.047).
3.3.3. Glutathione Peroxidase 1 (GPX1)
Fig. (7) presents the salivary GPX1 results. In Table 4, similar to SOD, no significant within-group changes in mean GPX1 levels were observed over the study period for either the Matcha tea group (Baseline: 221 ± 81.2 U/mL; Follow-up: 250 ± 81.2 U/mL; p = 0.100) or the control group (Baseline: 201 ± 78.3 U/mL; Follow-up: 203 ± 73.1 U/mL; p = 0.715). Nonetheless, the inter-group comparison at the one-month follow-up demonstrated that the Matcha tea group exhibited significantly higher mean salivary GPX1 levels than the control group (p = 0.027).
| Parameter | Group | n | Baseline Mean ± SD (U/mL) | Follow-up Mean ± SD (U/mL) | Mean Change (Follow-up - Baseline) ± SD (U/mL) | Within-Group p-value | Inter-Group p-value (Follow-up) |
|---|---|---|---|---|---|---|---|
| MDA | Matcha | 21 | 19.5 ± 5.2 | 15.6 ± 5.9 | -3.9 ± NA | 0.002** | 0.183 |
| Control | 20 | 13.4 ± 4.9 | 12.6 ± 3.4 | -0.8 ± NA | 0.476 | ||
| SOD | Matcha | 21 | 28.5 ± 15.0 | 31.9 ± 15.7 | +3.4 ± NA | 0.296 | 0.047* |
| Control | 20 | 24.8 ± 13.2 | 22.5 ± 9.4 | -2.3 ± NA | 0.498 | ||
| GPX1 | Matcha | 21 | 221 ± 81.2 | 250 ± 81.2 | +29 ± NA | 0.100 | 0.027* |
| Control | 20 | 201 ± 78.3 | 203 ± 73.1 | +2 ± NA | 0.715 |
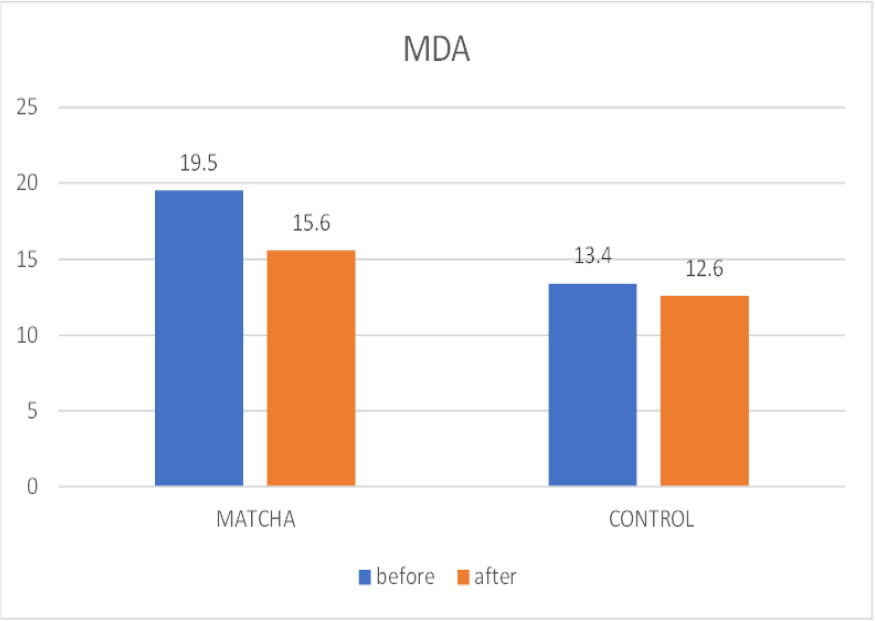
Mean MOD before and after intervention.
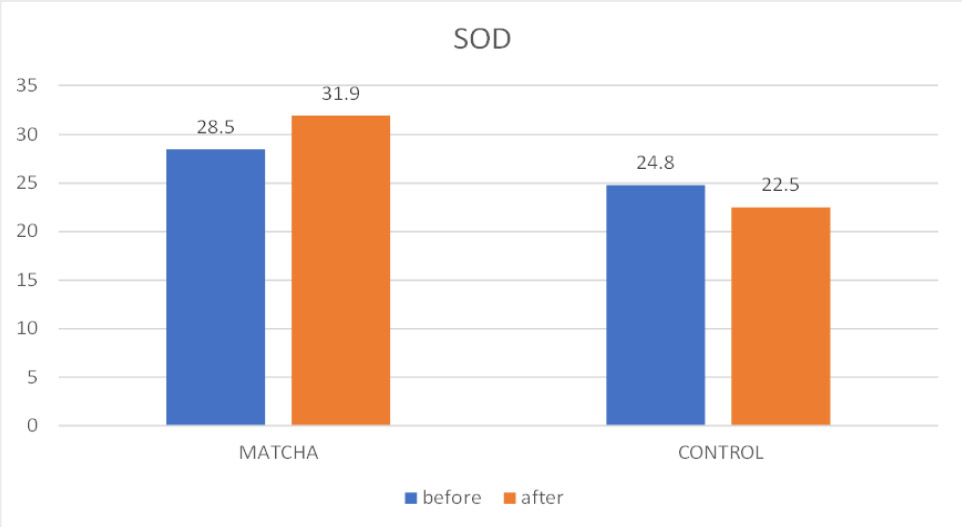
Mean SOD before and after intervention.
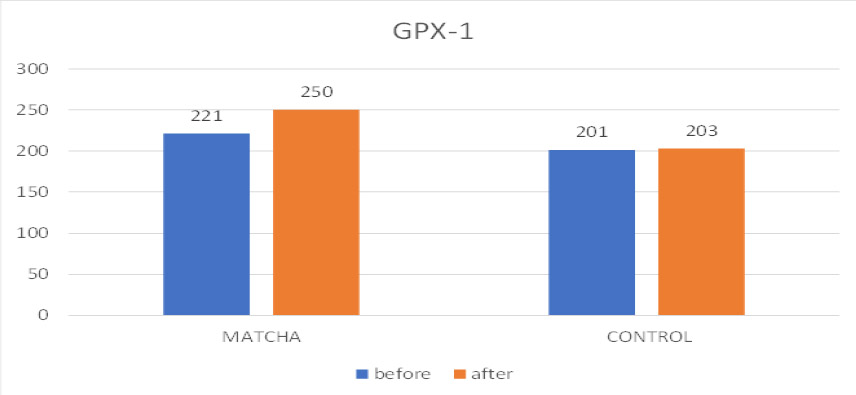
Mean GPX-1 before and after intervention.
| - | Age | PI (2) | BOP% (2) | GI (2) | MDA (2) | SOD (2) | GPX (2) |
|---|---|---|---|---|---|---|---|
| Age | 1 | 0.319* | 0.438** | 0.380* | 0.121 | -0.143 | -0.054 |
| PI (2) | 0.319* | 1 | 0.766** | 0.764** | 0.23 | 0.013 | -0.017 |
| BOP% (2) | 0.438** | 0.766** | 1 | 0.859** | 0.275 | -0.037 | 0.016 |
| GI (2) | 0.380* | 0.764** | 0.859** | 1 | 0.21 | -0.034 | -0.065 |
| MDA (2) | 0.121 | 0.23 | 0.275 | 0.21 | 1 | 0.201 | 0.370* |
| SOD (2) | -0.143 | 0.013 | -0.037 | -0.034 | 0.201 | 1 | 0.771** |
| GPX (2) | -0.054 | -0.017 | 0.016 | -0.065 | 0.370* | 0.771** | 1 |
 Strong positive (r ≥ 0.7)
Strong positive (r ≥ 0.7)
 Moderate positive (0.3 ≤ r < 0.7)
Moderate positive (0.3 ≤ r < 0.7)
 Weak positive (0 < r < 0.3)
Weak positive (0 < r < 0.3)
 Weak negative (-0.3 < r < 0).
Abbreviations: PI (2): Plaque Index at one-month flow-up, BOP%(2): Bleeding on Probing percentage at one-month flow-up; GI(2): Gingival Index at one-month flow-up; MDA(2) Malondialdehyde at one-month flow-up; SOD(1) Superoxide Dismutase at one-month flow-up; GPX1(2) Glutathione Peroxidase 1 at one-month flow-up; Significance levels: * p < 0.05, ** p < 0.01.
Weak negative (-0.3 < r < 0).
Abbreviations: PI (2): Plaque Index at one-month flow-up, BOP%(2): Bleeding on Probing percentage at one-month flow-up; GI(2): Gingival Index at one-month flow-up; MDA(2) Malondialdehyde at one-month flow-up; SOD(1) Superoxide Dismutase at one-month flow-up; GPX1(2) Glutathione Peroxidase 1 at one-month flow-up; Significance levels: * p < 0.05, ** p < 0.01.
3.4. Correlations
Spearman's rank correlation analysis was performed to examine relationships between clinical parameters and salivary biomarkers at one-month follow-up across all participants (n=41), regardless of group assignment. Strong positive correlation between antioxidant enzymes SOD and GPX1 (r = 0.771, p < 0.01), indicating coordinated antioxidant response; moderate positive correlation between MDA(2) and GPX1(2) (r = 0.370, p < 0.05), suggesting antioxidant upregulation in response to oxidative stress; and strong intercorrelations among clinical parameters PI(2), BOP%(2), and GI(2) (r = 0.764-0.859, all p < 0.01), confirming these measures assess related aspects of gingival inflammation. Age showed moderate positive correlations with clinical parameters (r = 0.319-0.438, p < 0.05). Notably, no strong correlations were observed between clinical and biochemical parameters, suggesting they measure different aspects of the disease process. The correlation matrix is presented in Table 5.
4. DISCUSSION
This randomized controlled clinical trial investigated the impact of daily Matcha tea consumption on both clinical periodontal parameters and salivary antioxidant profiles in individuals with dental biofilm-induced gingivitis. The most significant finding of this study is the demonstration that standard oral hygiene instruction and motivation represent the primary therapeutic intervention for gingivitis management, with both groups showing substantial and equivalent clinical improvements regardless of matcha tea consumption. While we observed significant within-group improvements in clinical indices (PI, BOP%, GI) in both the Matcha tea and control groups, highlighting the fundamental effectiveness of mechanical plaque control, the more intriguing findings emerged from the salivary biomarker analysis, where the Matcha tea group demonstrated specific biochemical changes that did not translate into additional clinical benefits within the study timeframe.
The comprehensive oral hygiene instructions provided to all participants, including proper brushing technique, interdental cleaning methods, and ongoing motivation, resulted in substantial reductions in plaque accumulation, gingival bleeding, and inflammation. The fact that generalized gingivitis was resolved entirely in both groups (from 30% and 48% at baseline to 0% at follow-up) demonstrates the powerful effect of optimized oral hygiene practices. It reinforces current periodontal therapy guidelines that emphasize mechanical biofilm control as the cornerstone of gingivitis treatment. This finding is consistent with decades of periodontal research demonstrating that gingivitis is reversible with adequate plaque control, and it provides essential context for interpreting the additional effects of matcha tea consumption.
The observed reduction in MDA in the Matcha group likely stems from the high concentration of polyphenolic compounds, particularly catechins like EGCG, present in Matcha tea. These compounds are potent free radical scavengers [51-53]. In gingivitis, the inflammatory response triggered by bacterial biofilm produces reactive oxygen species (ROS) by neutrophils and other immune cells [54]. Excessive ROS can overwhelm the endogenous antioxidant defense system, leading to oxidative damage, including lipid peroxidation reflected by elevated MDA levels [55-57]. Matcha's antioxidants may directly neutralize these ROS, thereby reducing the extent of lipid damage and lowering salivary MDA concentrations. This finding aligns with other in vitro and in vivo studies demonstrating the antioxidant capacity of green tea and its constituents in various biological systems [58]. For instance, studies have shown that green tea extracts can inhibit lipid peroxidation in cell cultures exposed to oxidative stressors [59].
The significant increase in salivary SOD and GPX1 levels in the Matcha group suggests a potential upregulation or protection of these key endogenous antioxidant enzymes. The coordinated increase in both enzymes, as evidenced by their strong positive correlation (r = 0.771, p < 0.01), suggests a systematic upregulation of antioxidant defense mechanisms. SOD catalyzes the dismutation of superoxide radicals to hydrogen peroxide and oxygen [60, 61], while GPX1 reduces hydrogen peroxide to water, using glutathione as a cofactor [62-64]. In gingivitis, the chronic oxidative stress can potentially deplete or impair the function of these enzymes [65-67]. The consumption of Matcha tea might provide bioactive compounds that either directly enhance the activity or expression of these enzymes or indirectly protect them from inactivation by ROS. Some studies have indicated that certain dietary antioxidants can influence the expression of antioxidant enzymes [68]. While our study did not directly measure enzyme activity, the increased protein levels detected by ELISA suggest a greater availability of these protective enzymes in the saliva of Matcha tea consumers. This observation contrasts with some studies in periodontitis patients that have reported decreased levels of salivary antioxidants, highlighting the potential for early intervention in gingivitis with antioxidant-rich substances like Matcha tea [69, 70].
The dissociation between the improved salivary antioxidant profile and the lack of statistically significant differences in clinical periodontal parameters between the groups could be attributed to several factors. As previously discussed, the relatively short intervention period and the powerful effect of improved oral hygiene in both groups might have masked the subtle clinical benefits of Matcha tea. Furthermore, while salivary biomarkers provide a valuable insight into the oral environment's redox status, they represent a global measure. They may not directly correlate with the gingival tissues' localized inflammatory and oxidative processes. Systematic reviews and meta-analyses of green tea interventions in periodontal therapy have shown mixed but generally positive results, with most studies reporting modest improvements in periodontal parameters when green tea is used as an adjunct to conventional therapy [71, 72]. However, many of these studies have used topical applications such as mouthwashes or subgingival irrigation, which may provide more direct local effects compared to systemic consumption [69]. Studies investigating systemic consumption of green tea for oral health have reported varying degrees of clinical improvement, with some showing significant benefits and others finding primarily biochemical changes similar to our findings [73, 74]. These inconsistencies might be due to differences in the form of tea consumed (extracts vs. whole tea), dosage, intervention duration, and the severity of gingivitis in the study populations.
The correlation analysis in our study further highlights the complex interplay between clinical inflammation and systemic/oral antioxidant status. The lack of strong correlations between clinical parameters and salivary biomarkers at the one-month follow-up suggests that while Matcha tea can modulate the salivary antioxidant environment, the direct translation of these changes into readily measurable clinical improvements in gingivitis requires further investigation, potentially with longer studies and more localized measures of oxidative stress. The significant positive correlations between MDA and GPX1, and between SOD and GPX1, could reflect a coordinated endogenous antioxidant response attempting to counteract the ongoing oxidative stress, with GPX1 potentially being upregulated in response to increased lipid peroxidation products and both enzymes working synergistically to neutralize different ROS.
This study provides evidence that daily Matcha tea consumption can favorably modulate salivary oxidative stress biomarkers in individuals with gingivitis by reducing lipid peroxidation and increasing the availability of key antioxidant enzymes. While these biochemical benefits did not manifest as statistically significant differences in clinical periodontal parameters compared to optimized oral hygiene within the 30-day study period, the findings suggest a potential adjunctive role for Matcha tea in supporting oral health by enhancing antioxidant defense mechanisms. Future research with longer intervention durations, larger sample sizes, and potentially the assessment of more localized oxidative stress markers is warranted to elucidate further Matcha tea consumption's clinical significance in managing gingivitis and its potential to prevent the progression to more advanced periodontal diseases.
5. LIMITATIONS
The study's findings should be interpreted with consideration of several limitations, including the relatively small sample size, which may have limited statistical power, the short one-month intervention period, which might not have allowed for full clinical manifestation of biochemical changes, and the study population consisting of individuals with mild to moderate gingivitis, potentially limiting generalizability.
CONCLUSION
This study demonstrates that both the Matcha tea and control groups achieved significant improvements in the primary outcomes (clinical periodontal parameters: Plaque Index, Bleeding on Probing percentage, Gingival Index) at the one-month follow-up (p<0.01). However, there were no statistically significant differences in these primary clinical parameters between the two groups at the endpoint. For secondary outcomes, daily consumption of matcha tea for one month significantly improved salivary antioxidant status in individuals with dental biofilm-induced gingivitis, as demonstrated by a reduction in the lipid peroxidation marker MDA and an increase in the levels of the antioxidant enzymes SOD and GPX1 compared to the control group. The most important finding of this study is the demonstration that proper oral hygiene instruction and motivation represent the primary and most effective intervention for gingivitis management, with both groups achieving substantial and equivalent clinical improvements regardless of matcha tea consumption. The complete resolution of generalized gingivitis in both groups underscores the fundamental importance of mechanical plaque control in periodontal therapy.
AUTHORS’ CONTRIBUTIONS
The authors confirm contribution to the paper as follows: A.T.Y. and S.S.S.: Conceptualization, Methodology; A.T.Y., S.S.S. and S.M.I.: Validation; Formal analysis; Writing—original draft preparation, Writing—review and editing; A.T.Y.: Investigation, Resources, Data curation; A.T.Y.: Visualization; S.S.S. and S.M.I.: Supervision. All authors have read and agreed to the published version of the manuscript.
LIST OF ABBREVIATIONS
| BOP% | = Bleeding on Probing percentage |
| GI | = Gingival Index |
| GPX1 | = Glutathione Peroxidase 1 |
| MDA | = Malondialdehyde |
| OS | = Oxidative Stress |
| PI | = Plaque Index |
| ROS | = Reactive Oxygen Species |
| SOD | = Superoxide Dismutase |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Ethics approval was obtained from the Ethics Committee of the College of Dentistry, University of Baghdad, Iraq (Ref. number: 883623, dated 3/12/2023).
HUMAN AND ANIMAL RIGHTS
No animals were used in this research. All procedures performed in studies involving human participants were in accordance with the ethical standards of institutional and/or research committee and with the 1975 Declaration of Helsinki, as revised in 2013.
CONSENT FOR PUBLICATION
Written informed consent was obtained from all participants prior to their enrollment in the study. Participants were informed of their right to withdraw from the study at any time without justification.
AVAILABILITY OF DATA AND MATERIALS
The data supporting the findings of the article will be available from the corresponding author [S.M.I] upon reasonable request.
ACKNOWLEDGEMENTS
We appreciate the assistance and resources provided by the Department of Periodontics at the Kufa College of Dentistry, Iraq.


