All published articles of this journal are available on ScienceDirect.
The Use of Ozone Therapy in Combination with a Desensitizing Agent for Dentinal Tubules Occlusion: An In Vitro Study
Abstract
Introduction
A new method for reducing dentin hypersensitivity is ozone therapy. This study aimed to evaluate the efficacy of ozone therapy combined with a desensitizing agent in reducing dentin hypersensitivity.
Methods
Sixty samples of dentin sections were distributed randomly into 3 groups as group 1 (control group) with 20 samples, group 2 (Gluma group) with 20 samples. The discs were coated with Gluma. Moreover, for group 3 (Gluma + O3 group) with 20 samples, the samples were treated with ozone therapy followed by treatment with Gluma. Scanning Electron Microscopic (SEM) views of the dentin specimens were acquired, and the average tubule occlusion amount was recorded in each case employing a suitable scoring system.
Results
The control group demonstrated no completely occluded tubules, while the Gluma group showed a mean occlusion of 33.39% (±13.83). The highest level of complete occlusion was observed in the Gluma + O3 group, where the mean percentage reached 80.87% (±10.31). The Gluma group had a mean partial occlusion of 5.17% (±4.11), and the Gluma + O3 group showed a higher mean partial occlusion of 6.33% (±4.25).
Discussion
Since 20% of the dentin is formed from organic matrix, which is mainly composed of collagen, different studies have shown that degradation of collagen bonds can occur due to the oxidative action of O3. Therefore, ozone can reduce dentin sensitivity by mechanically blocking the dentinal tubules.
Conclusion
Compared to applying the Gluma agent alone, it was discovered that ozone treatment resulted in a more compact deposition of the Gluma desensitizer particles.
1. INTRODUCTION
Dentin hypersensitivity is a dental condition that is considered one of the most prevalent and challenging dental problems [1]. In recent decades, considerable attention has been focused on individual health and quality of life, resulting in healthy and functional dentition, even in older age [2]. Consequently, it is anticipated that there will be a dramatic increase in dentin hypersensitivity among elderly people in our population. Today, this condition impacts approximately 35% of the population around the world, primarily affecting individuals aged 20 to 50 in the community [3]. Furthermore, it seems to affect females in higher numbers compared to males [4]. Previous studies showed that 72% of patients with gingival recession and 98% who have periodontal disease are suffering from dental hypersensitivity [5]. Dentin hypersensitivity is scientifically defined as a short episode of sharp and severe pain that arises from exposed dentin. Usually, the administration of chemical, thermal, tactile, or osmotic stimuli typically initiates the pain, which cannot be caused by any other oral pathology or defect [6]. However, this kind of dental condition can impair an individual’s quality of life. This leads to a significant deterioration in patients’ daily activities, including drinking, eating, speaking, and even toothbrushing [7]. Difficulty during dental brushing makes it uncomfortable for individuals to maintain good oral health. However, this kind of discomfort is highly subjective and usually can vary from patient to patient [8]. Dentin hypersensitivity can be treated either by desensitizing the nerve or blocking the dentinal tubules [9]. Agents containing oxalate salts, strontium, and fluoride are among the common dentin-occluding agents [10-12]. The study by Talwar et al. demonstrated the remineralization of enamel and dentin lesions using three different fluoridated dentifrices (with fluoride concentrations of 2500 ppm, 5000 ppm, and 1100 ppm) using microradiography and an Electric Caries Monitor. They showed that higher fluoride levels did not result in superior remineralization of enamel or dentin. Nevertheless, their treatment approach led to a decrease in dentin sensitivity using fluoride regardless of the concentration [13]. Although various treatment modalities have been proposed to control the condition, current treatments for tooth hypersensitivity remain inadequate [11, 14, 15]. Ozone therapy has been suggested as a novel approach for treating dentin hypersensitivity, especially after bleaching [16]. Previous studies have suggested that ozone ions can act as anti-inflammatory and antioxidant agents, which can reduce inflammation and sensitivity [6, 17, 18]. According to a study by McKenna et al. ozone therapy alone or in combination of hydrogen peroxide reduced gingival bleeding and plaque scores in the soft tissues of patients with peri-implant mucositis. Moreover, their results showed that gaseous ozone could delay the advancement of peri-implant diseases, thereby preventing tearing of the tissue [19]. Despite the growing evidence of literature supporting the use of ozone therapy in various dental applications, its role in managing dentin hypersensitivity remains undetermined and insufficiently validated. Existing studies have primarily focused on ozone's antimicrobial and anti-inflammatory properties, with limited emphasis on its potential as a desensitizing agent. Therefore, this study seeks to evaluate the synergistic effect of ozone application in combination with a conventional desensitizing agent. By investigating its impact on dentin hypersensitivity, this research aims to introduce a novel, minimally invasive therapeutic approach that could enhance patient outcomes and broaden the clinical utility of ozone therapy in dentistry.
2. MATERIALS AND METHODS
This in vitro study was conducted in the Department of Periodontics, the College of Dentistry, Prince Sattam bin Abdulaziz University, Alkharj. Approval was obtained from the Standing Committee of Bioethics Research (SCBR) with No. (SCBR-075-2023). Human permanent upper and lower premolar teeth extracted during orthodontic therapy in the orthodontic department were used in this study. Written informed consent was obtained from the patients for using their teeth, and the project was conducted according to the regulations of the Declaration of Helsinki, which was revised in 2013 by the World Medical Association. Teeth with clinically noticeable cracks, dental caries, stains, erosive/abrasive regions, attrition, and white spot lesions were excluded from the study. The extracted premolars were cleaned using periodontal curettes and an ultrasonic cleaner (TM-5LK, Foshan TopMed Dental Co., Ltd., China). Subsequently, followed by prophylaxis with a rubber cup and pumice. The teeth were stored in a weekly renewed phosphate-buffered solution (PBS) at 37 °C until use.
2.1. Sample Size
To estimate the required sample size, a post-hoc power analysis was conducted using G*Power for a one-way ANOVA comparing three independent groups on a single occasion. The computation was based on an anticipated effect size (f) of 0.60, which represents a medium-sized effect. The alpha level was set at 0.05, and the study included 60 measurements, with twenty measurements per group. The resulting power reached 0.93, comfortably above the conventional threshold of 0.80, indicating that the sample size of 20 per group is adequate for detecting moderate to large group differences after covariate adjustment.
2.2. Preparation of Dentin Blocks
At the level of the Cementoenamel Junction (CEJ), the collected teeth were sectioned using a two-sided diamond disk in a horizontal direction using a water-cooled mechanism (Struers, Copenhagen, Denmark). A round bur (ISO 005) (Komet 0197, GmbH, Besigheim, Germany) was then used on the enamel side to create a central indentation until the dentin was exposed at the standardized depth. A water-cooled mechanical grinder (Red-wing, A.C. motor, Handler Co., Westfield, New Jersey, USA) was then employed to grind the enamel down to the depth of the indentation. In order to create blocks that were about 2 mm thick and were taken from the cervical third of the buccal halves of teeth, the dentin discs were then ground on a 200-grit carbide plate to remove any remaining enamel on the occlusal side and the pulp horn on the pulp side of the disc. They were then polished using 400, 800, and 1200 grit carbide polishing papers [20, 21]. For the opening of dentinal tubules, the blocks were subjected to ultrasonication using distilled water for 10 minutes, and 35% phosphoric acid was used for 30 seconds to etch the dentin, thereby completely exposing the dentinal tubules. After that, distilled water was used to rinse the samples. The specimens were immersed in Phosphate‐Buffered Solution (PBS) till future utilization.
2.3. Group Allocation
The specimens were randomly assigned to 3 groups of 20 samples each. In group 1, the samples were rinsed with saline for one minute (control). In Group 2, the Gluma group, small cotton pellets were used to apply a small amount of Gluma desensitizer (Heraeus Kulzer; CA, USA) from a small bottle to the dentin discs, according to the manufacturer's instructions. The discs were then left for 20–40 seconds. The surface was then dried with a stream of compressed air until the film of fluid was gone and the surface was no longer shiny. It was then washed with water several times. Group 3 samples were treated with ozone therapy first, then Gluma. The samples were treated with ozone gas, which was delivered as pure oxygen from a cylinder connected to the generator (HealOzone X4 machine OTM-3125; Curozone, Germany) and set to a flow rate of 1L/min for 6 minutes, as suggested by the International Ozone Association (https://www.ioa-pag.org/). This provided the dentin samples with a high concentration of ozone (100,000 to 300,000 ppm). The investigator involved in sample preparation and treatment application was blinded to group allocation to minimize bias.
2.4. Sample Preparation for Scanning Electron Microscopy
The dentin discs were fixed on stubs and coated with gold by sputtering for subsequent SEM analysis. Subsequently, SEM pictures were captured at a magnification of 5000×. After observing the sample's image under SEM, the images were evaluated independently by two evaluators who were blinded and well trained to score the level of tubule occlusion based on Saini’s study according to the following categories:
Type 0: Exposed dentinal tubules.
Type 1: Partial occlusion of dentinal tubules, affecting less than 25% of the orifice.
Type 2: Partial occlusion of dentinal tubules above 25% and up to 75% of the dentinal tubule orifice.
Type 3: Substantial occlusion of dentinal tubule orifices, exceeding 75% of the tubule orifice.
During the evaluation, types 1 and 2 were classified within the same category as partial dentinal tubule occlusion [22]. The percentage of occluded tubules was calculated by dividing the total number of occluded dentinal tubules by the total number of dentinal tubules in the SEM image. The result was subsequently multiplied by 100 to derive the percentage of occluded dentinal tubules for each image, as outlined in a prior study. The same procedure was applied to determine the proportion of partially occluded tubules [23].
2.5. Statistical Methods
Mean differences between groups were calculated, along with p-values to determine statistical significance. A p-value of less than 0.05 was considered significant for all tests. For comparison of means between the study groups, a One-Way Analysis of Variance (ANOVA) was conducted, followed by post-hoc testing using Tukey’s test to assess significant differences in dentinal tubule occlusion between the groups. The primary analysis focused on comparing the percentage of complete and partial occlusion of dentinal tubules across the control, Gluma, and Gluma + O3 groups.
The reliability analysis for this study used several key methods to assess the relationships and reliability of measurements across the different treatment groups. The Spearman’s rank correlation coefficient (Spearman r) was used to evaluate the strength and direction of the relationship between two sets of repeated readings. Additionally, to assess the agreement and reliability of the measurements, the Intraclass Correlation Coefficient (ICC) was calculated, indicating the degree of consistency or reliability across repeated readings.
3. RESULTS
Table 1 demonstrates the measurements of the dentinal tubules and occlusion percentages across the study groups. Regarding complete occlusion of dentinal tubules, the control group showed no occluded tubules, whereas the Gluma group showed a mean occlusion percentage of 33.39% (±13.83). The highest level of complete occlusion was observed in the Gluma + O3 group, where the mean percentage reached 80.87% (±10.31). Median values followed a similar pattern, with the Gluma group at 33.20% and the Gluma + O3 group at 81.82%. The partial occlusion of dentinal tubules was also examined. The control group exhibited no partial occlusion (0%), while the Gluma group had a mean occlusion of 5.17% (±4.11), and the Gluma + O3 group showed a higher mean occlusion of 6.33% (±4.25). Median values were 4.41% for the Gluma group and 6.25% for the Gluma + O3 group, suggesting an increase in partial occlusion with the addistion of ozone treatment. For SEM examination of the surface topography, Fig. (1) shows the morphology of dentinal tubules treated with saline as a control, in which almost all the dentinal tubules were opened, and no occluded dentinal tubules were present. In Fig. (2), the image shows a relatively smooth thin surface coating over some areas of the treated surface with Gluma. On the other hand, the surface topography of the Gluma + O3 specimen revealed a thick layer of coating, which was hiding most orifices of the dentinal tubules, and some tubules were barely visible (Fig. 3). The results of the multiple comparisons in Table 2 show the effects of different treatments on dentinal tubules. For the number of dentinal tubules, no significant differences were found between the groups. This indicates that the study groups were comparable, so variations in the dentinal tubule count do not confound any observed differences in dentinal tubule occlusion. On the other hand, the percentage of complete occlusion of dentinal tubules showed significant differences between study groups. Both Gluma and Gluma + O3 treatments resulted in significantly higher mean percentages of dentinal occlusion compared to the control group, as the control group had a zero occlusion percentage (p-value <0.001). In partial occlusion, statistically significant differences were also detected in the comparisons between the control group, Gluma group, and the Gluma + O3 group, as the control group had a zero dentinal occlusion. However, the comparison between the Gluma Group and Gluma + O3 did not show a significant difference (p = 0.533), suggesting that both treatments had similar effects on partial occlusion.
| Measurement | - | Control | Gluma | Gluma+O3 |
|---|---|---|---|---|
| Number of total dentinal tubules | (Mean ± SD) | 31.90 ± 3.29 | 30.85 ± 4.53 | 28.98 ± 5.05 |
| Median (Range) | 31.50 (11.0) | 32.50 (15.0) | 28.75 (16.0) | |
| Percentage of Complete Occlusion of dentinal tubules | (Mean ± SD) | 0 | 33.39 ± 13.83 | 80.87 ± 10.31 |
| Median (Range) | 0 | 33.20 (45.51) | 81.82 (40.30) | |
| Percentage of Partial Occlusion of dentinal tubules | (Mean ± SD) | 0 | 5.17 ± 4.11 | 6.33 ± 4.25 |
| Median (Range) | 0 | 4.41 (13.89) | 6.25 (14.93) |
| Measurement | Comparison | Mean Difference | p-value | 95% Confidence Interval |
|---|---|---|---|---|
| Number of total dentinal tubules | Control Group vs Gluma Group | 1.05 | 0.727 | -2.26, 4.36 |
| Control Group vs Gluma + O3 | 2.93 | 0.094 | -0.39, 6.24 | |
| Gluma Group vs Gluma + O3 | 1.88 | 0.368 | -1.44, 5.19 | |
| Percentage of Complete Occlusion of dentinal tubules | Control Group vs Gluma Group | -33.39* | <0.001 | -40.97, -25.81 |
| Control Group vs Gluma + O3 | -80.87* | <0.001 | -88.45, -73.29 | |
| Gluma Group vs Gluma + O3 | -47.48* | <0.001 | -55.06, -39.90 | |
| Percentage of Partial Occlusion of dentinal tubules | Control Group vs Gluma Group | -5.17* | <0.001 | -7.76, -2.57 |
| Control Group vs Gluma + O3 | -6.33* | <0.001 | -8.93, -3.73 | |
| Gluma Group vs Gluma + O3 | -1.16 | 0.533 | -3.76, 1.44 |

Morphology of dentinal tubules treated with saline (control), seen under scanning electron microscope (× 5,000).

Morphology of dentinal tubules treated with (Gluma), seen under scanning electron microscope (× 5,000).
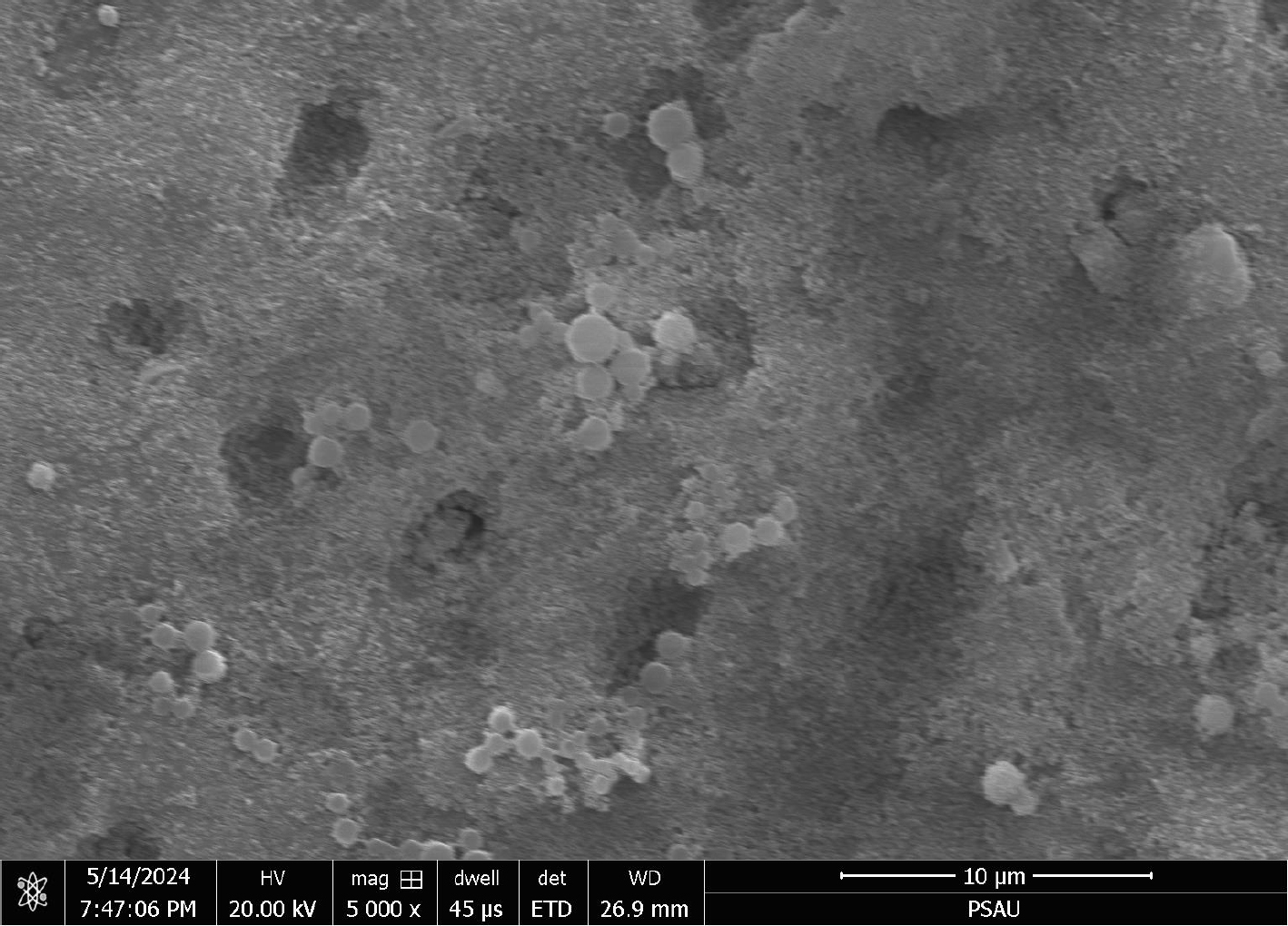
Morphology of dentinal tubules treated with (Gluma+ O3), seen under scanning electron microscope (× 5,000).
| Measurement Pair | Spearman Correlation Coefficients | ICC Estimate | ICC Lower CI | ICC Upper CI | BA Mean Diff | BA SD | LOA Lower | LOA Upper |
|---|---|---|---|---|---|---|---|---|
| Control group | ||||||||
|
Number of total dentinal tubules (Readings 1 vs. Readings 2) |
0.959 | 0.92 | 0.81 | 0.968 | 0.1 | 1.373 | -2.59 | 2.79 |
| Gluma group | ||||||||
|
Number of total dentinal tubules (Readings 1 vs. Readings 2) |
0.863 | 0.891 | 0.746 | 0.956 | 0.01 | 2.224 | -4.360 | 4.360 |
|
Complete Occlusion of dentinal tubules (Readings 1 vs. Readings 2) |
0.925 | 0.916 | 0.802 | 0.966 | -0.5 | 1.732 | -3.895 | 2.895 |
|
Partial Occlusion of dentinal tubules (Readings 1 vs. Readings 2) |
0.735 | 0.802 | 0.565 | 0.917 | -0.1 | 0.968 | -1.997 | 1.797 |
| Gluma+ O3 group | ||||||||
|
Number of total dentinal tubules (Readings 1 vs. Readings 2) |
0.938 | 0.907 | 0.784 | 0.962 | 0.35 | 2.254 | -4.068 | 4.768 |
|
Complete Occlusion of dentinal tubules (Readings 1 vs. Readings 2) |
0.983 | 0.978 | 0.931 | 0.992 | 0.50 | 0.889 | -1.242 | 2.242 |
|
Partial Occlusion of dentinal tubules (Readings 1 vs. Readings 2) |
0.877 | 0.862 | 0.667 | 0.944 | -0.30 | 0.657 | -1.588 | 0.988 |
Regarding the reliability assessment, Table 3 presents the reliability of repeated readings across the three study groups. In the control group, the total dentinal tubules, with a Spearman r of 0.96 and an ICC estimate of 0.92, indicated excellent reliability between the two readings, characterized by a minimal Bland-Altman mean difference of 0.1 and a standard deviation of 1.37. The limits Of Agreement (LOA) ranged from -2.59 to 2.79, indicating good consistency between the repeated measurements. In the Gluma group, the Spearman correlation coefficient of the measurement of total dentinal tubules was 0.863, and the ICC estimate was 0.891, indicating good reliability. The Bland-Altman mean difference was 0.01, with a larger standard deviation of 2.224, and the LOA ranged from -4.360 to 4.360. For complete occlusion of dentinal tubules, the measurement showed a Spearman r of 0.93, and the ICC estimate was 0.916, showing excellent consistency between the readings. In the Gluma + O3 group, the Spearman r of 0.94 and ICC estimate of 0.91 indicate good reliability, with a Bland-Altman mean difference of 0.35 and a standard deviation of 2.25. The LOA ranged from -4.07 to 4.77. For complete occlusion measurement, the Spearman r was 0.98, and the ICC estimate of 0.98 showed near-perfect reliability.
4. DISCUSSION
Dentin sensitivity is a serious condition that interferes with patients' daily tasks and standard of living. According to a recent systematic review in 2024, treatments for teeth sensitivity were found to enhance the psychological dimension of comfort by reducing perceived pain [24], thereby justifying the relevance of conducting further research in this field. The present in vitro investigation was undertaken to assess the effect of applying ozone gas in combination with commercially available Gluma agent on dentinal tubule occlusion. In this study, dentin blocks were prepared from the buccal surface of premolar teeth at the area of the cervical third, which is a frequent site of dentin hypersensitivity due to the presence of a thin layer of enamel [25, 26]. The dentin blocks were treated for 2 minutes with 35% phosphoric acid to guarantee adequate removal of the smear layer and dentinal plugs to display the underlying tubules, thereby clinically simulating the hypersensitivity of dentin [27]. Moreover, it is well known that 86% of the total resistance to the movement of dentinal fluids is because of the accumulation of the smear layer [28]. However, the primary strategy to reduce dentin hypersensitivity is by focusing on substances that target dentin permeability or interdental nerve blockage, whether by precipitating a material directly into the majority of tubules and/or forming a layer over the top of the exposed dentin [11, 18, 29]. Consequently, the dentin will become insensitive to stimuli such as cold drinks or airflow, which, in normal conditions, can stimulate the nerve endings that induce pain [12, 30, 31]. Results from the present study showed that ozone therapy was significantly effective in complete occlusion of dentinal tubules compared to Gluma and the control group. This agrees with a previous in vitro study by Saha et al., which evaluated the effect of ozonated oil and ozone gas with and without the application of desensitizing toothpaste on dentinal tubule occlusion. In their experiment, the maximum score of total tubular occlusions before and after the acid challenge was for the toothpaste and ozonated oil group [32].
In this study, O3 gas was delivered from a HealOzone x4 machine, which is an activation generator for pure oxygen with high voltage and frequency power to deliver a maximum concentration of oxygen for a 6-minute duration according to the International Ozone Association [33]. When it comes in contact with the dentin blocks for this duration, it emits energy and splits the environmental dioxygen into unique ozone and a single oxygen particle. Such a condition will lead to the temporary recombination of oxygen atoms into groups of three [21, 34, 35]. An in vitro study by Rasha et al. found that the combined use of fluoride with ozone treatment resulted in a significantly wider dentin tubule diameter compared to the control. Moreover, they explained that the oxidizing property of ozone was 5 times greater than that of oxygen [21].
According to earlier investigations, the ions of ozone possess an antioxidant with an anti-inflammatory effect, due to prolonged reduction in prostaglandin secretion and inactivation of cyclooxygenase, which will lead to the suppression of inflammatory pathways [21, 36]. Additionally, since 20% of the dentin is formed from the organic matrix, which is mainly composed of collagen, different studies have shown that degradation of collagen bonds can occur due to the oxidative action of O3. Therefore, by collagen degradation, ozone can reduce dentin sensitivity by mechanically blocking the dentinal tubules [17, 37]. Besides that, considering its gaseous condition, O3 was believed to diffuse in a more efficient way than other desensitizer agents throughout the tooth structure [6]. A clinical study by Detogni et al. evaluated the effect of an experimental ozone-based gel on dentin sensitivity and a high percentage of bleaching material. They found that the experimental gel containing O3 is a promising desensitizing agent that can reduce tooth sensitivity, without interfering with the color achieved by dental bleaching [34]. Nevertheless, the powerful oxidizing quality of ozone might contribute to the management of sensitivity, but with a different mode of action. Abdelaziz et al. indicated that the smear layer can be removed when the exposed dentin has been subjected to ozone application. Later, the dentinal tubules will be broadened in diameter, facilitating the entrance of minerals such as calcium and fluoride ions from saliva or desensitizing agents [21]. For this reason, it has been suggested that ozone application should not be used alone for treating tooth sensitivity, but could be considered a possible adjunct to fluoride-containing desensitizers in enhancing tubular occlusion [17, 38]. In comparison with other desensitizing agents, Sathish et al. investigated the effectiveness of Casein Phosphopeptide‐Amorphous Calcium Phosphate (CPP‐ACP) tooth mousse and fluoride‐doped amorphous calcium phosphate (F‐ACP) on the occlusion of dentinal tubules. They found that among the two, F‐ACP was significantly greater in dentin occlusion [39]. However, it is difficult to compare their findings with these results since the physical action of ozone is quite different and was suggested as a potential auxiliary treatment to reduce sensitivity [9]. Glutaraldehyde (Gluma desensitizer) in this study is more effective in dentinal tubule occlusion compared to saline. Furthermore, this differed from the results reported by Joshi et al., who compared the impact of calcium sodium phosphosilicate (NovaMin) and Gluma in blocking dentinal tubules. They demonstrated that both agents were effective in occluding dentinal tubules, but NovaMin showed greater potential in obstructing them completely following initial application [40]. Thus far, the results from this study have been consistent with previous experimental in vitro studies by Mushtaq et al. and Jiang et al., which have demonstrated the favourable efficacy of Gluma in relieving dentin hypersensitivity [41, 42]. At the same time, these findings suggested that the adjunctive use of ozone therapy before the application of the Gluma agent can lead to more compact and heavy deposition of the Gluma particles compared to the application of the latter alone. A possible synergistic effect was noted, where O3 caused the opening of dentinal tubules, allowing for more penetration of the desensitizing agent. In line with that, previous studies confirmed a significant increase in the diameter of dentinal tubules resulting from the highly reactive and unstable ozone atoms, which are unable to maintain their body structure over time [35]. Consequently, when the O3 comes into contact with unsaturated compounds such as carbohydrates and proteins on the dentin surface, it will induce a chemical reaction. This reaction enlarges the diameter of the dentinal tubules, facilitating the entry of minerals from saliva or desensitizing agents into these tubules [9, 43]. Although this study did not evaluate the longevity of treatment and resistance over time, a previous study by Amario et al. showed that ozone gas, compared to the efficacy of diode laser in the treatment of dentin sensitivity, both modalities had a significant decrease immediately and after 3 and 6 months of treatment. Additionally, Ozone therapy maintains great effectiveness of hypersensitivity reduction after 6 months [35].
5. LIMITATIONS AND FUTURE RECOMMENDATIONS
This study has several limitations, the first being the in vitro design, as samples were not stored in artificial saliva or treated under dynamic oral conditions such as saliva flow and pH changes, which may not accurately reflect in vivo tubule occlusion stability or desensitizing efficacy. Despite these limitations, efforts were made to replicate oral cavity conditions and strictly adhere to the manufacturer’s guidelines for material application. The second limitation of this study is that the depth of desensitizing agents and penetration into the dentinal tubules were not evaluated. Moreover, the resistance of sealed tubules, occlusion resistance testing, and variability in tubule counts were not determined. Additionally, the small sample size of 20 per group may be insufficient to detect significant differences between groups. Future long-term studies with a larger sample size and different mechanical testing methods, such as occlusion resistance and depth of material penetration, are needed to explore the physical findings in dentinal tubules. Furthermore, combinations of therapies or different application techniques can enhance the effectiveness of ozone therapy. Longitudinal randomized clinical trials with patient-reported pain outcomes are needed to corroborate the findings obtained in the present in vitro study.
CONCLUSION
In conclusion, ozone treatment was found to produce a more compact deposition of the particles of the Gluma desensitizer compared to the application of the Gluma agent alone. There was a statistically significant difference between the two groups, and a comparison with the control revealed a significant difference in the ratio of partial and complete occlusion. With these observations, the use of ozone therapy may provide appreciable clinical therapeutic benefits before the application of a desensitizing agent, which can lead to effective management of dentinal hypersensitivity.
AUTHOR’S CONTRIBUTIONS
The author confirms sole responsibility for the following: study conception and design, data collection, analysis and interpretation of results, and manuscript preparation.
LIST OF ABBREVIATIONS
| SEM | = Scanning Electron Microscopic |
| CEJ | = Cementoenamel Junction |
| PBS | = Phosphate‐Buffered Solution |
| ANOVA | = One-Way Analysis of Variance |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The study was authorized by the Standing Committee of Bioethics Research at the dental college, Prince Sattam bin Abdulaziz University, Saudi Arabia (SCBR-075-2023).
HUMAN AND ANIMAL RIGHTS
All human research procedures followed were in accordance with the ethical standards of the committee responsible for human experimentation (institutional and national), and with the Helsinki Declaration of 1975, as revised in 2013.
CONSENT FOR PUBLICATION
Informed written consent was obtained from the patients for the use of their extracted teeth.
AVAILABILITY OF DATA AND MATERIALS
The data and supportive information are available within the article.
FUNDING
This study is supported via funding from Prince Sattam bin Abdulaziz University project number (PSAU/2024/R/1446).
ACKNOWLEDGEMENTS
Declared none.


