All published articles of this journal are available on ScienceDirect.
Genotoxicity and Cytotoxicity of Cone Beam Computed Tomography Compared to Panoramic and Bitewing Radiographs in Children
Abstract
Introduction
Dental radiographs play a crucial role in diagnosis. However, radiation has a cumulative effect on children and may lead to carcinogenesis. To determine the genotoxicity and cytotoxicity in buccal mucosal cells of children exposed to panoramic and bitewing radiographs or Cone Beam Computed Tomography (CBCT) at various time intervals.
Methods
A cohort study was conducted involving 38 healthy children (ages 6-12), divided into two groups: one group received panoramic and bitewing radiographs, and the other underwent CBCT. Buccal mucosal cells were collected at baseline, and follow-ups occurred at 10±2 days, one month, and three months post-exposure. Cells were stained using Feulgen/fast green and analyzed microscopically for genotoxic (micronuclei) and cytotoxic (condensed chromatin, karyolysis, pyknosis, karyorrhexis) markers.
Results
At the 10±2 days mark, an increase in micronuclei was observed in both groups, with no significant difference (p-value=0.660). However, after one month, the percentage of micronuclei was significantly lower in the CBCT group compared to the first group (p-values < 0.001). Cytotoxic alterations showed a comparable pattern, with transient increases in both groups that returned to baseline at three months.
Discussion
The findings indicate transient and reversible genotoxic and cytotoxic effects in buccal mucosal cells due to radiographic exposure. While CBCT exhibited lower micronuclei counts after one month, both modalities showed similar patterns of cellular damage.
Conclusion
These results highlight the importance of adhering to the ALARA principle in pediatric dentistry, as the observed effects of dental radiography are transient and do not indicate long-term damage. Further research is recommended to explore these effects in larger populations.
1. INTRODUCTION
Dental radiographs are a frequently used tool in contemporary dental practice, essential for accurate diagnosis and treatment planning [1, 2]. Each of these imaging techniques must be chosen according to the principle of As Low As Reasonably Achievable (ALARA), ensuring the image benefit does outweigh the risk of radiographic exposure [3]. This is of great importance in pediatric dentistry, due to the increased radiosensitivity in children resulting from their rapidly growing tissues. In addition, the potential risk of radiation-induced tumors through the cumulative effects of ionizing radiation in children may be higher [4].
The diagnosis of proximal caries has traditionally been performed using bitewing radiographs, which support clinical examination, especially when clinical visual inspection alone is insufficient due to tight interproximal contacts [5-8]. Conversely, panoramic radiographs are popular for their pediatric applications as they are commonly used to assess dental development, craniofacial trauma, and to facilitate treatment planning [9, 10]. Currently, Cone-Beam Computed Tomography (CBCT) has become widely used in most dental specialties, particularly to provide adequate diagnostic information for complex cases that cannot be achieved with conventional radiographs [11-16].
Evaluating both genotoxicity and cytotoxicity in buccal mucosal cells is a helpful approach to assessing the biological effects of radiation exposure. Micronuclei are small nuclei that can form when chromosomal damage occurs, and they are well-established biomarkers of genotoxicity [17]. Furthermore, other nuclear alterations, including binucleation, karyolysis, pyknosis, and karyorrhexis, are also important markers of cytotoxic damage, albeit less specific than micronuclei [17-19]. At the same time, bitewing and panoramic radiographs have been shown to increase the frequencies of micronucleated cells in exfoliated buccal mucosa, with bitewings exhibiting a more genotoxic effect than panoramic radiographs [20-23]. On the other hand, progressive decrease in micronuclei may be visible even in newly formed conjunctiva during and after long archived radiographies, however, their association to these paths has not yet described with molecular arrangement like other parameters including pyknosis, karyorrhexis, and karyolysis by the small doses of different detectors, on the contrary, there is direct association between specific established conditions [24-29]. Interestingly, conventional panoramic radiographs cause a higher increase in micronuclei than digital panoramic systems [23]. However, panoramic exposure did not show any statistically significant difference in the frequency of micronuclei between children and adults, indicating that children may not be more susceptible to radiation-induced damage [25].
Carlin et al. (2010) and Lorenzoni et al. (2013) reported the documentation of various cytotoxic features, such as karyolysis, pyknosis, and karyorrhexis, in buccal mucosal cells post-CBCT exposure, with no increase in the frequency of micronuclei observed in cells 10 days post-exposure [30, 31]. However, the genotoxic and cytotoxic effects of panoramic radiographs and CBCT on exfoliated buccal mucosal cells in children have not been explored yet. This study was therefore conducted to determine the genotoxicity and cytotoxicity in buccal mucosal cells of children exposed to panoramic, bitewing, and CBCT radiographs at various time intervals (10±2 days, 1 month, and 3 months). The null hypothesis was that there is no significant difference in genotoxicity or cytotoxicity in the exfoliated buccal mucosal cells of children after exposure to these imaging modalities.
2. MATERIAL AND METHODS
This prospective cohort study was conducted at King Abdulaziz University, Faculty of Dentistry (KAUFD), between January 2019 and December 2020, as a continuation of the research by Altoukhi (2021) et al. [32]. Ethical approval was obtained from the Research Ethics Committee of the Faculty of Dentistry (REC-FD) at King Abdulaziz University (145-11-18).
The sample size was calculated to achieve a medium effect of 10 subjects per group using G*Power software (Version 3.1.9.3) (HHU, Germany) at an 80% power and 0.05 significance level. The number of subjects per group was increased to 20 to compensate for any dropouts.
During the study period, new pediatric patients visiting the Pediatric Dentistry Screening Clinic at KAUFD were screened for eligibility by the study investigator. The inclusion criteria included 6- to 12-year-old healthy children with no history of head and neck radiation within the last six months and with good to fair oral hygiene, as assessed by the debris score part of the simplified oral hygiene index [33]. They had a justified clinical indication for panoramic radiographs or CBCT. All the radiographs taken for the included subjects in the study had a justified clinical indication, and no radiographs or CBCT were taken solely for research purposes, as was done in Altoukhi et al.'s previous study [32]. Any patient who did not meet these criteria was excluded from the study.
The study investigator introduced the study aims to the parents/guardians of eligible children. Those who agreed to participate were asked to sign an Arabic consent form, and an appointment was scheduled to obtain the required panoramic and bitewing radiographs or CBCT. At the scheduled appointment, the medical history, along with age, gender, and nationality, was recorded. In the first group, the participating subjects required panoramic and two-bitewing radiographs. In the second group, a CBCT was needed to evaluate the tendency of permanent maxillary canine impaction due to mesioangular inclination observed on their panoramic radiographs taken within the last 6-12 months, as described in the Altoukhi et al. study [32].
The panoramic radiographs were performed by a trained radiologist using the Gendex Digital Panoramic X-Ray System (GXDP-700TM, United States) with 66 kv, 4 mA, and 14 seconds. The total effective dose was around 6.55 μSv [34]. The Bitewing radiographs were taken using a Gendex Intraoral X-Ray System (Gendex, United States) with a 65 kV and 7.5 mA setting, and a 0.16-second exposure time. The total effective dose was about 1.7 μSv [35]. For the CBCT, an i-CAT CBCT scanner was used (Kavo Kerr, United States) with (FOV) 16×6 cm, 120 kv, 10 mA, 4.8 seconds, and 0.4 voxels on the maxillary canine area. The effective dose was approximately 22 μSv [36], as mentioned in the previous research by Altoukhi et al. [32].
Following the methodology of Altoukhi et al.'s 2021 study, a clinical examination appointment was scheduled 10±2 days later with a trained pediatric dentist. The participating subjects were instructed to thoroughly rinse their mouths with water to remove debris before obtaining the cells. Exfoliated buccal mucosal cells were obtained from each participating subject by scraping the buccal mucosa on both the right and left sides multiple times using a Rovers® special brush (BD, Netherlands). The same method was repeated after one and three months after the exposure. The scraped cells were then collected in sample bottles containing BD SurePathTM Preservative Fluid (Ethanol, Methanol, and Isopropanol) (BD, Ireland) [32].
The Cytological preparations were performed in the Cytology lab at King Abdulaziz University Hospital (KAUH). First, the samples were placed in centrifuge tubes (3400 rpm) for three minutes (Hettich, Germany), followed by the removal of the supernatant layer. A manual cell counting chamber (Lafontaine, Belgium) was used to remove one thousand cells from each sample to be stained and scored for genotoxicity and cytotoxicity. The sample was then placed between two charge slides (Thermo Scientific, United States) and fixed immediately in 95% Ethanol (Honeywell, USA) for 20 minutes. Then, a DNA-specific stain (Feulgen/light green (Bio-Optica, Italy) was used and examined under a light microscope [32]. Genotoxicity (micronuclei) and cytotoxicity (condensed chromatin, karyolysis, pyknosis, and karyorrhexis) were scored following the criteria explained by Tolbert et al. in 1992 [17].
Each slide was examined by a trained and calibrated pediatric dentist and pathologist independently for the presence of:
1. The existence of the main nucleus with another smaller nucleus or nuclei describes a Micronucleus.
2. Condensed chromatin, which shows nuclei with aggregated chromatin regions with a speckled nuclear pattern. Chromatin accumulates in some areas of the nucleus but disappears in other regions.
3. Karyorrhexis is a more extensive chromatin aggregation seen in the nucleus of karyorrhectic cells, causing fragmentation and degeneration.
4. Pyknosis, in which the cells are presented with shrunken nuclei that have high-density and uniformly stained nuclear material.
5. Karyolysis, in which the cells are entirely diminished of DNA in the nucleus with no Feulgen staining, leads to a ghost-like cell appearance.
For the cytological analysis, the exfoliated buccal cells from each participating subject were examined using a light microscope (Olympus, Japan) with a 400X magnification, equipped with a digital camera (SC 180) (Olympus, Japan) and connected to a computer (Dell, USA). A cellSens imaging software (Olympus, Japan) was used to capture and save the images for scoring. The frequency of genotoxicity and cytotoxicity changes was counted in 1,000 cells for each participating subject in each study period, following the same methodology used in the Altoukhi et al. study [32].
Both examiners were blinded to group allocation of the participating subjects being examined and the period after exposure (10±2 days, one and three months). The intra-examiner and inter-examiner reliability after training using Cohen's kappa coefficient test (κ) on SPSS software (IBM, NY, version 22) was examined by examining 16 slides by both examiners. The same slides were examined again after two weeks [32].
2.1. Statistical Analysis
The Mann-Whitney U test was applied to compare the percentages of participating subjects with genotoxic and cytotoxic changes at the three different periods: (10±2 days, 1 month, and 3 months). The level of significance was set at p-values < 0.05. SPSS version 22 (IBM Corp., 2017) was used for the statistical analysis.
3. RESULTS
The study included 38 participants, split into two groups: 20 subjects received panoramic and bitewing radiographs, and 18 participants received CBCT. The average age of the subjects receiving panoramic and bitewing radiographs was 8.4 ± 2.1 years, which was significantly lower (p-values < 0.001) than the average age (11.0 ± 0.9 years) of the subjects who received CBCT. The sex distribution between the groups showed no significant difference (p-values = 0.203). The demographic characteristics of the participating subjects are presented in Table 1
| - | Panoramic and Bitewings Radiographs n=20 | CBCT n=18 | p-value | |
|---|---|---|---|---|
| Age in years | Mean ± SD | 8.4 ± 2.1 | 11.0 ± 0.9 | <0.001* † |
|
Sex n (%) |
Male | 13 (65.0) | 8 (44.4) | 0.203 € |
| Female | 7 (35.0) | 10 (55.6) | ||
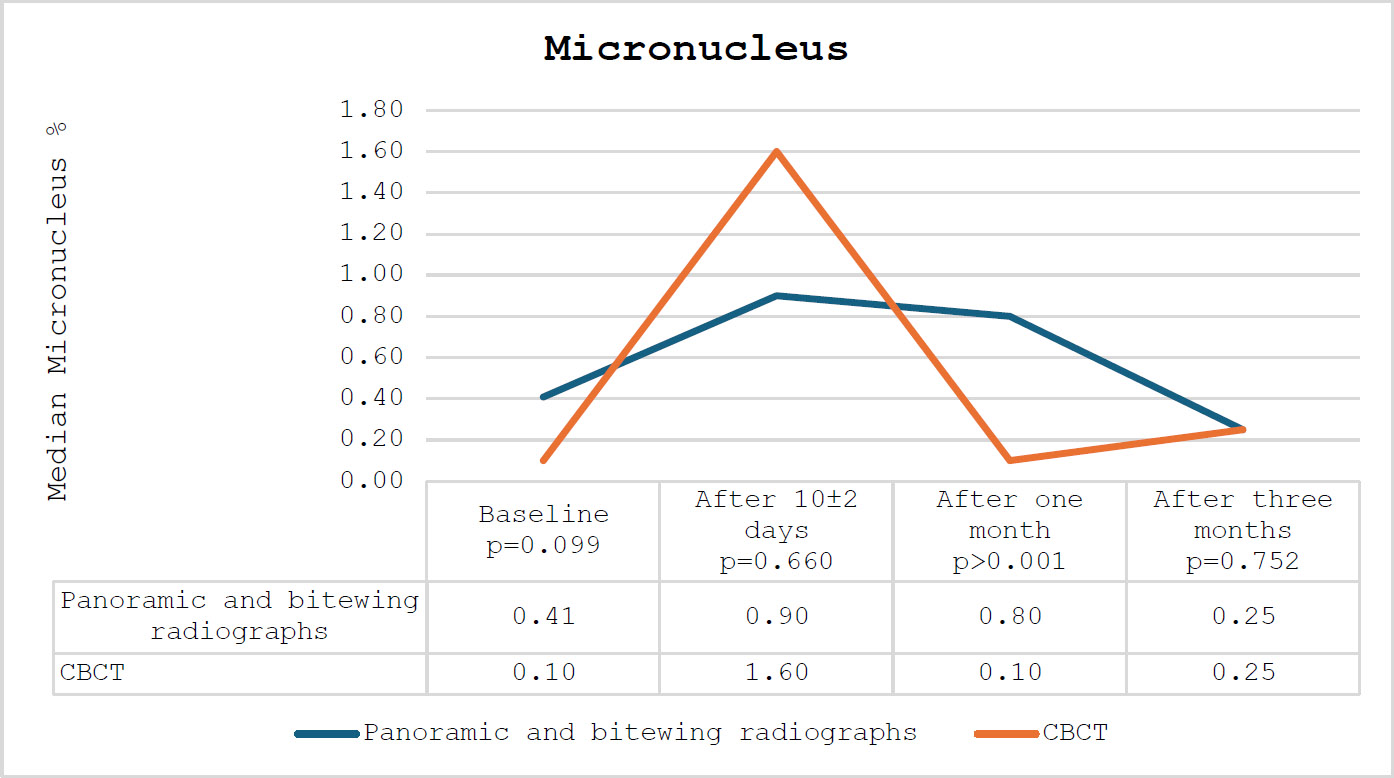
The percentage of micronuclei among the participating subjects.
The CBCT groups exhibited a more pronounced increase in the percentage of micronuclei after 10±2 days relative to the panoramic and bitewing radiographs groups; however, this difference did not reach statistical significance (p-value=0.660). A notable decline was observed in the CBCT group compared to the panoramic and bitewing radiographs group. The percentage of micronuclei in the CBCT group was significantly lower compared to the panoramic and bitewing radiographs after one month (p-values < 0.001). After three months, the percentage of micronuclei returned to baseline levels. The percentage of micronuclei among the participating subjects is presented in Fig. (1).
In relation to condensed chromatin and karyorrhexis, a comparable pattern was noted among the participating subjects: an increase after 10±2 days, succeeded by a decrease after one and three months. Overall, the percentages of participating subjects in the CBCT group exceeded those in the panoramic and bitewing radiographs group. The percentages of condensed chromatin and karyorrhexis among the participating subjects are presented in Figs. (2 and 3), respectively.
The percentage of karyolysis increased in both groups after 10±2 days, followed by a steady decline in both groups after one and three months. The percentage of karyolysis among the participating subjects is presented in Fig. (4).
Finally, regarding pyknosis, the CBCT group exhibited significantly elevated levels after 10±2 days (p-values = 0.004) and after one month (p-values = 0.006) compared to the panorama and bitewing groups. After three months, the levels in both groups were comparable (p-values = 0.796). The percentage of pyknosis among the participating subjects is presented in Fig. (5).
4. DISCUSSION
The findings of this study help explore the genotoxic and cytotoxic effects of different types of dental radiographic imaging on buccal mucosal cells of child patients.
These results are consistent with existing studies and suggest areas for further investigation. In the CBCT group, there was a transient peak of micronuclei at 10±2 days post-exposure, which decreased significantly by one month and returned to baseline levels by three months. It suggests transient genotoxic effects due to CBCT, which are reversible over time. In fact, previous investigations reached the same conclusion as the present one, finding significant genotoxic effects 10 ± 2 days after exposure to CBCT [37]. However, none of the previous research studies followed up more than 10 ± 2 days after CBCT exposure, indicating that these results are of great value and recommend further research with extended follow-up periods and a larger sample size to evaluate the residual effects of CBCT.
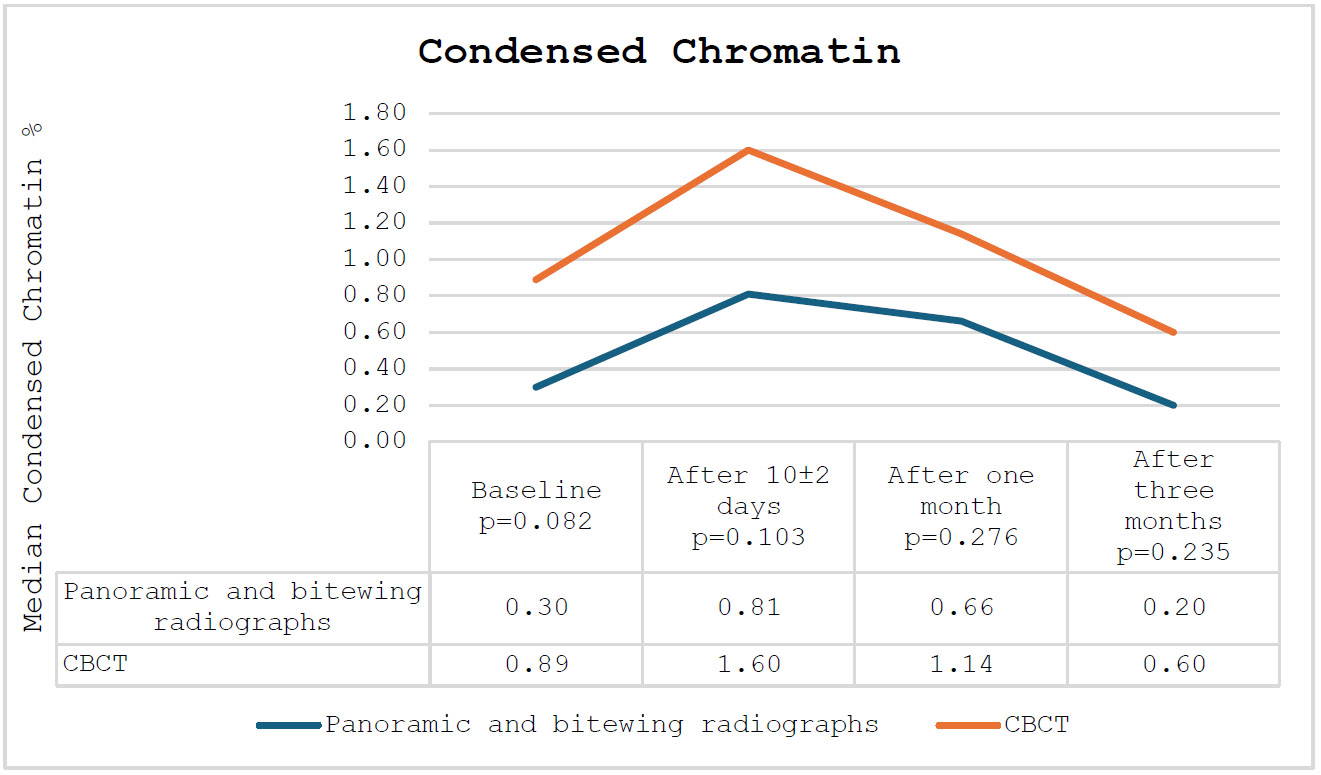
The percentage of condensed chromatin among the participating subjects.
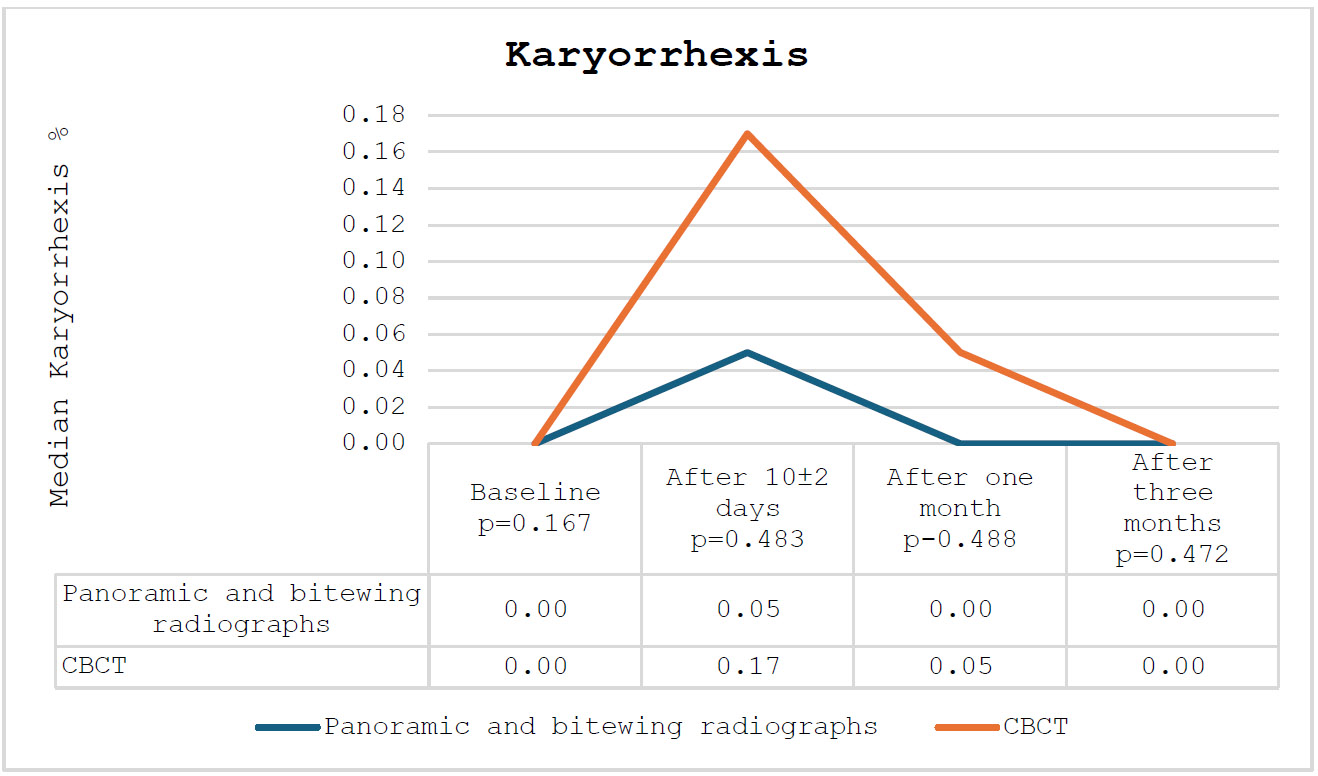
The percentage of karyorrhexis among the participating subjects.
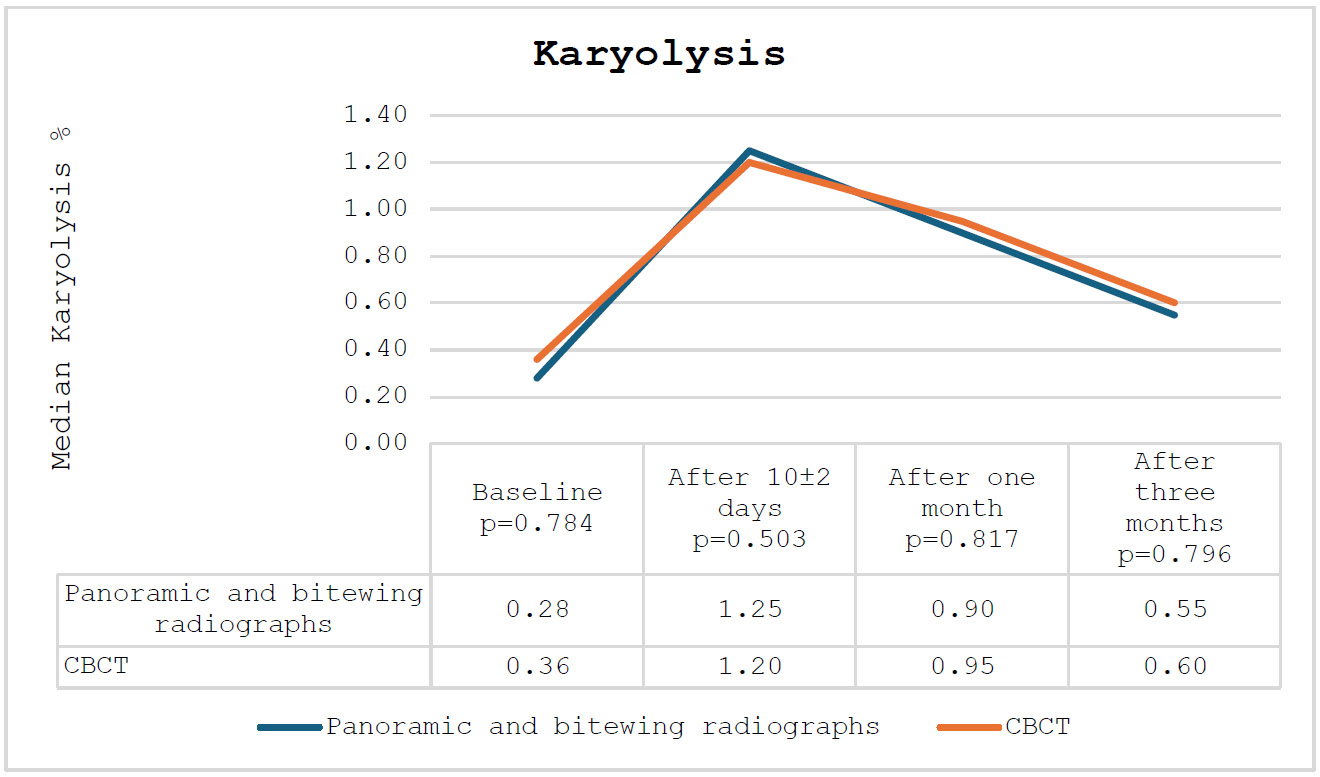
The percentage of karyolysis among the participating subjects.
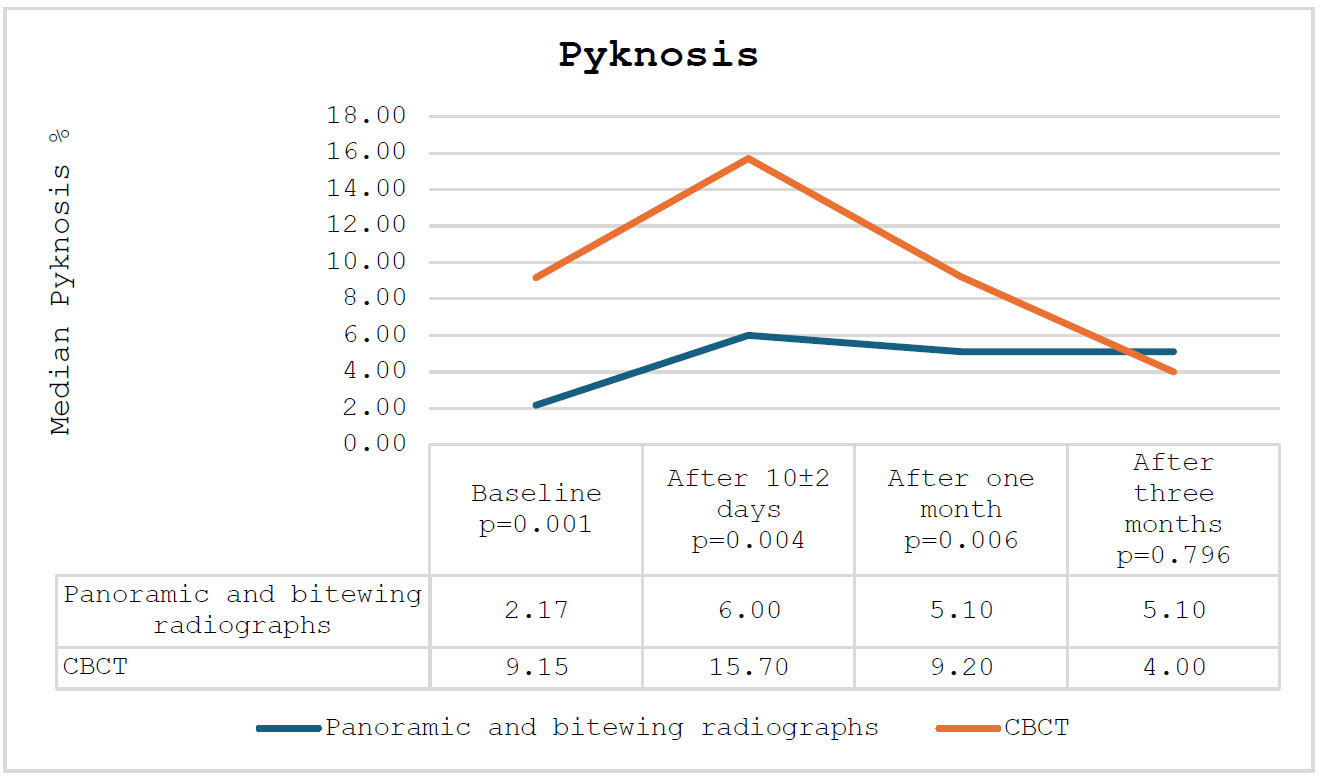
The percentage of pyknosis among the participating subjects.
The finding that there was no major disparity in the frequency of micronuclei between the CBCT group and control radiographs (bitewing and panoramic) at 10±2 days (p-value=0.660) would lead them to suggest that the use of CBCT may not pose a clinically relevant higher level of genotoxic hazard than standard diagnostic processes. This finding aligns with the results of Aggarwal et al. (2019), which also reported a statistically significant increase in the frequency of micronuclei in the buccal mucosa of children 10 ± 2 days after digital panoramic radiography [21]. However, a considerable decrease in the frequency of micronuclei in the CBCT group after 1 month (p-values < 0.001) also implies that cellular repair mechanisms effectively remove radiation-induced damage over time.
In this study, the increase in cytotoxic changes (condensed chromatin, karyorrhexis, and karyolysis) was greater in the CBCT group compared to the panoramic and bitewing groups. This finding agreed with other studies, which published similar cytotoxic outcomes after exposure to CBCT, including karyolysis and pyknosis [30, 31]. The increased number of cytotoxic changes observed in the CBCT group post-exposure (p-values = 0.004 and p-values = 0.006, respectively) suggests that CBCT may be more cytotoxic than conventional radiographs. However, since the levels returned to normal within three months, it would be a reversible effect.
The cytotoxicity effects described in this study are in accord with those reported by Angelieri et al. (2010) [27], which are believed to be due to panoramic radiographs causing nuclear changes such as pyknosis and karyorrhexis. Likewise, Vinuth et al. report that traditional panoramic X-ray imaging causes more cytotoxic damage than digital technology [28]. These changes are reversible, suggesting that cell remediation mechanisms effectively counteract the cytotoxicity associated with radiation.
To understand cellular repair mechanisms, it is essential to recognize that the epithelium covering the oral mucosa relies on stem cells for tissue regeneration [38]. It is well known that normal tissue stem cells create a lifelong reserve of cells capable of self-regeneration. Division of epithelial cells occurs mainly in the basal layer, which contains stem cells. After division of the basal cells, they undergo differentiation as cells move superficially and shed off the surface. The progenitor stem cells of the oral epithelium take approximately 7–16 days to divide and cross throughout the full thickness of the oral epithelium (epithelial turnover time) [39]. The Expression of many stem cell markers, such as CD44, Keratin 14, Bmi1, and Sox2, has been mainly identified in the basal layer, indicating that it is considered a reserve of stem cells. Nevertheless, the mechanisms of maintenance and regeneration of these cells remain unknown [40].
There was a significant difference in the mean age of the subjects between the CBCT group (11.0 ± 0.9 years) and the panoramic and bitewing groups (8.4 ± 2.1 years) (p-values < 0.001). Still, the fact that no significant difference was found in the frequency of micronuclei and other nuclear changes between the age groups agrees with Ribeiro et al.'s 2008 results, confirming a lack of genotoxic and cytotoxic differences between children and adults exposed to the same type of panoramic imaging [25]. Moreover, a recent study by Anbumeena et al. (2021) conducted on five different age groups, including children, adolescents, young Adulthood, and late adulthood, found no significant differences between age groups regarding the genotoxic and cytotoxic effects of panoramic radiography [41].
The study emphasizes the need for adherence to the ALARA (As Low As Reasonably Achievable) principle in the selection of radiographic imaging modalities for pediatric patients. Furthermore, bitewing, panoramic, and CBCT radiographs have reversible genotoxic and cytotoxic effects, which are transient and seem to be without long-term hazards. On the other hand, according to the American Academy of Oral and Maxillofacial Radiology's recommendation, given the larger cytotoxic effects produced by the CBCT group, the use of this technique should be limited to areas where conventional radiographs fail to provide the necessary diagnostic information [16].
The fact that the effects are transient also reflects the effectiveness of cellular repair systems in suppressing radiation-induced harm. This is particularly reassuring in the case of children's dentistry, where doses of radiation must be kept low, as developing tissue is more susceptible to damage by radiation [4].
5. LIMITATIONS
Although this study presents useful insights, it has some limitations. Since the sample sizes are low, the results may be slightly biased in favor of the younger participants in the CBCT and panoramic/bitewing groups. In addition, larger sample sizes and age-matched controls should be used in future studies to confirm the findings. The study also assessed buccal mucosal cells, which are not necessarily an indicator of radiation's effect on other tissues. The cytotoxic and genotoxic outcomes of dental radiography may also be further studied, not only in epithelial cells but also in other cell lines, such as salivary gland or gingival cells.
CONCLUSION
In conclusion, dental radiography (CBCT, panoramic, and bitewing) results in transient genotoxicity and cytotoxicity on buccal mucosal cells of children with no indication of long-term harm. Even though the effects appear to be reversible, adhering to the ALARA principle remains of high importance in pediatric dental imaging.
AUTHORS’ CONTRIBUTIONS
The authors confirm their contributions to the paper as follows: S.A., D.A., and E.E.: Study conception and design; D.A.: Collected the data; O.N. and O.F.: Conducted the analysis and interpretation of the results; S.B., A.A., and H.B.: Prepared the draft manuscript. All authors reviewed the results and approved the final version of the manuscript.
LIST OF ABBREVIATIONS
| CBCT | = Cone Beam Computed Tomography |
| ALARA | = As Low As Reasonably Achievable |
| KAUFD | = King Abdulaziz University, Faculty of Dentistry |
| REC-FD | = Research Ethics Committee of the Faculty of Dentistry |
| KAUH | = King Abdulaziz University Hospital |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The study received ethical approval from the Research Ethics Committee of the Faculty of Dentistry (REC-FD) at King Abdulaziz University, Saudi Arabia (145-11-18).
HUMAN AND ANIMAL RIGHTS
All procedures performed in studies involving human participants were in accordance with the ethical standards of institutional and/or research committee and followed the 1975 Declaration of Helsinki, as revised in 2013.
AVAILABILITY OF DATA AND MATERIALS
The data supporting the findings of the article is available at King Abdulaziz University, Saudi Arabia drive (https://www.kau.edu.sa/ar).
ACKNOWLEDGEMENTS
Declared none.


