All published articles of this journal are available on ScienceDirect.
The Safety of Licorice Extract Gel for Potential Use in Oral Care
Abstract
Introduction
Herbal medicine offers alternative ways to combat antimicrobial resistance in the treatment of human diseases. Licorice (Glycyrrhiza glabra) is a medicinal plant with anti-inflammatory, antimicrobial, and antioxidant properties, which has been increasingly explored in dentistry for its potential benefits in managing oral diseases. The aim of this in vitro study was to assess the cytotoxicity of licorice extract gel against Human Oral Fibroblast (HOrF) cells for potential use in oral and dental care.
Methods
The gel was formulated by incorporating ethanolic licorice extract into a carbomer-based gel base. To measure cytotoxicity, HOrF cells were used. Cytotoxicity was assessed by measuring the number of live cells at 24, 48, and 72 h time intervals. The plate containing HOrF cells without any substance was considered the control group.
Results
The results showed that the licorice extract gel (100 µg/mL) did not exhibit cytotoxicity on HOrF cells at the experimentally tested time points. Statistical analysis showed that there was no significant difference in the cytotoxicity of licorice extract gel (100 µg/mL) between the control and test group at any time of 24, 48, and 72 h.
Discussion
Licorice extract gel at 100 µg/mL was non-toxic to human oral fibroblasts, suggesting its safety for oral applications. Fibroblasts are vital for oral tissue maintenance and wound healing, and the gel’s biocompatibility supports its potential use in oral care products. Licorice bioactive compounds, including glycyrrhizin and glabridin, offer anti-inflammatory, antimicrobial, and antioxidant benefits. While these results are promising, further studies, including animal models and clinical trials, are needed to confirm safety and evaluate long-term use. The results suggest that licorice extract gel could be a promising natural alternative for oral care products, though further studies are needed to confirm its clinical effectiveness.
Conclusion
Due to the non-toxicity of licorice extract gel (at 100 µg/mL) on HOrF, other oral formulations can be prepared from this material in the studied concentration. It is worth noting that the clinical use of this substance requires further animal and human investigations.
1. INTRODUCTION
Periodontal diseases are classified as the presence of periodontium inflammation caused by certain microorganisms that lead to the progressive destruction of periodontal tissue and bone. Periodontitis has two main causes. One of them is the accumulation of periodontopathogenic bacteria in the gum pocket, while another factor is the host's response to periodontal pathogenic bacteria [1]. In addition to periodontal disease, oral conditions such as dental caries, oral mucositis, halitosis, and candidiasis are common health concerns [2].
Local treatment of periodontal diseases is more practical due to the destruction pattern and limited damage in periodontal infections. On the other hand, topical drugs provide effective concentrations in the periodontal pocket and do not have the side effects of systemic drug use [3]. To increase the accumulation of an agent at the site of action, a suitable carrier is needed to deliver the antimicrobial agent in a controlled manner and provide the appropriate concentration and effectiveness in the environment. Carbapol is used as a mucoadhesive polymer, and its integration leads to increased mucosal contact time and improved formulation content [4]. This formulation is used in various biomedical preparations due to its known properties, such as biocompatibility, mucosal adhesion, antimicrobial biodegradability, and wound healing. In general, the medicinal form of oral gel can be associated with a slower effect but with a longer duration [4].
Different traditional medicines have existed in many countries around the world, including those of the Indian subcontinent, such as India, Pakistan, and Bangladesh, and continue to be derived from more than 7,500 plant species. Documenting and investigating the effectiveness of these medicinal substances with the scientific method is very important because a number of important modern medicines are derived from plants used by native people [5]. Herbal medicine offers alternatives to combat antibiotic resistance in the treatment of human diseases. There is a lot of evidence to prove the use of plants for the prevention and treatment of human diseases [6-8]. Herbal remedies, due to their bioactive phytochemicals, have shown promising effects in reducing oral pathogens, modulating inflammation, and enhancing tissue healing. Numerous studies have evaluated plant-based products such as neem, clove, tea tree oil, and miswak for their roles in oral hygiene maintenance and disease prevention [2, 3].
Licorice (Glycyrrhiza glabra L. (G. glabra)] is a traditional medicinal plant that grows in different parts of the world [9]. Due to its sweet taste, licorice is used worldwide as a sweetener and a flavoring agent in the production of food and medicine, and in the United States of America (USA) it is classified as 'generally safe' by the Food and Drug Administration (FDA) [10]. Licorice is rich in secondary metabolites that have been associated with various health benefits. Secondary metabolites of licorice root have a beneficial effect in the treatment of various diseases such as cancer, tuberculosis, atherosclerosis, peptic ulcer, immunodeficiency, hepatitis, and bacterial infections [7, 10].
Recently, the benefits of licorice in oral diseases have received much attention. Clinical trials have been conducted worldwide to evaluate the effects of licorice and its metabolites in the prevention and treatment of various oral diseases such as dental caries, periodontal diseases, candidiasis, aphthous ulcers, and debilitating diseases such as oral cancer. In addition, licorice has also been studied as a root canal medicine that can prevent unsuccessful root canal treatments and lead to more effective treatments [8, 11].
The capacity of licorice to minimize plaque formation turns it into a suitable candidate in the management of periodontitis. Bioactive plant compounds of G. glabra inhibit the growth of periodontopathogenic pathogens and reduce the expression of inflammatory markers at the site of infection. It also terminates the osteoclastic activity that contributes to the destruction of alveolar bone in periodontitis, whereas it strengthens the synthesis of osteoblasts to form new bone [12]. Besides, licorice root compounds with anti-adhesion properties can have favorable antimicrobial and anti-inflammatory effects in the oral cavity [12]. Licorice can act as a strong stimulant of saliva secretion, a natural mechanism to prevent oral caries [13]. Some studies have reported its effect on preventing the growth of Streptococcus mutans, a known cause of tooth decay [14-16]. The aim of this in vitro study was to assess the cytotoxicity of licorice extract gel against Human Oral Fibroblasts [HOrF] for potential use in oral and dental care.
2. METHODS
2.1. Study Design
This study was an in vitro experimental laboratory study designed to evaluate the cytotoxicity of licorice extract gel on Human Oral Fibroblasts (HOrF). The study followed these key design elements:
2.2. Study Type: In vitro Experimental
2.2.1. Objective
This study aimed to assess the cytotoxicity of a licorice extract gel for potential use in oral care.
2.2.3. Test Substance
Licorice extract formulated as a gel at a concentration of 100 μg/Ml was used.
2.3. Sample Size
Considering that the intervention is performed on the same samples and under the same laboratory conditions, three repetitions were performed for each variable. Therefore, in the 96-well plate, three wells were allocated to each group (N = 9). The sample size was approved by the ethics committee of Tabriz University of Medical Sciences.
2.4. Preparation of Licorice Extract Gel
Licorice samples were identified by a botanist (Ph.D. in Botany with expertise in medicinal plants) for their authenticity. After washing, all the samples were dried and powdered for 3 days. The obtained powder was then weighed up to 50 grams and mixed with 100 ml of sterile distilled water in a round-bottom flask with occasional shaking. Then, the extract was filtered through a muslin cloth to remove the coarse residue and finally through Whatman No. 1 filter paper, and stored in a closed, amber-colored container. To prepare the gel, the obtained 1% extract was depressurized in 100 mL of water and stirred on a magnetic stirrer at 500 rpm and a temperature of 30 °C. Afterward, 0.1% of the total weight of Tween 80 surfactant was added to the mixture, and it was stirred again for one hour under the mentioned conditions. Subsequently, 1%w/w of carbomer powder was added to the mixture as a gelling base material. After stirring the final material with a glass stirrer for 10 minutes, licorice extract [100 µg/mL] was prepared in the form of an oral gel. Finally, the prepared gel was sterilized by gamma [7].
2.5. Cytotoxicity Test
Human Oral Fibroblast (HOrF) cells were purchased from Shahid Beheshti University of Medical Sciences (Tehran, Iran) as characterized cells.
Evaluation of cytotoxicity was performed using the MTT method. At first, cells were prepared in culture medium enriched with 10% fetal bovine serum, streptomycin (50 μg/ml), and penicillin (50 units/ml) in an incubator with 5% CO2, a temperature of 37°C, and humidity of 95%. Under sterile conditions, a confluent cell flask was placed under the hood. The supernatant was removed, and the cells were washed with PBS. Trypsin was then added to detach the fibroblasts, causing them to change from an elongated to a spherical shape. Then, the medium enriched with 10% FBS was added to neutralize the added trypsin.Additionally, the cells were transferred into a tube and centrifuged at 1200 rpm for 5 min, allowing them to be completely separated from the culture medium and placed at the bottom of the tube. After centrifugation, the supernatant from the cells was discarded, and the culture medium was enriched with 10% FBS. The cells were then counted using a neobar slide. After culturing the cells and counting them, 30,000 cells were poured into each well of a 96-well plate and placed in a culture medium containing serum in a CO2 incubator for 24 h, until the cells adhered to the bottom of the wells. According to the standard ISO 10993-5, after 24, 48, and 72 h, the supernatant of the cells was removed, and the cell layer was washed with PBS, and MTT (1 mg/ml) solution (Sigma-Aldrich) was applied. The culture plate was again placed in a dark place for 5 h, and after the required time, the cells were washed again with PBS to remove the unreacted MTT with isopropanol solvent (Sigma-Aldrich). An Elizarider device at a 540 nm wavelength was used to measure the cell viability.
The cell viability was calculated based on the following equation:
The percentage of viable cells= [Average absorbance of control samples/Average absorbance of treated samples] ×100
2.6. Statistical Analysis
SPSS software (version 21) was used for statistical analysis. The results of the studies were reported using descriptive statistical methods. Subsequently, the normality of the data was tested using the Kolmogorov-Smirnov test, and finally, the two-way ANOVA test was used to compare the test and control groups at three time intervals [24, 48 and 72 h]. A p-value of equal to or less than 0.05 is considered significant.
2.7. Ethical Considerations
This study was conducted by obtaining the code of ethics from the Ethics Committee of Tabriz University of Medical Sciences [Ethics number: IR.TBZMED.VCR. REC.1402.074]. Human Oral Fibroblast (HOrF) cells were purchased from Shahid Beheshti University of Medical Sciences (Tehran, Iran) as characterized cells.
3. RESULTS
3.1. Cytotoxicity Results
The cytotoxicity results of licorice extract gel (100µg/mL) against HOrF cells showed no toxicity compared to the control group at 24, 48, and 72 hours (Fig. 1).
According to the two-way ANOVA test, there was no significant difference between the control and test groups at any time (24, 48, and 72 h) (p=0.5).
4. DISCUSSION
Cytotoxicity results of licorice extract gel against HOrF cells showed no toxicity for this formulation at the studied time points. Statistical analysis showed that there was no significant difference between the control and test groups at any time (24, 48, and 72 h).
Licorice is a medicinal plant with useful and diverse chemical compounds, and for this reason, it is of great importance in the food and pharmaceutical industries. A triterpene glycoside called glycyrrhizin is obtained from this plant, which is sweet and is used instead of sugar in some countries, such as Japan [17].
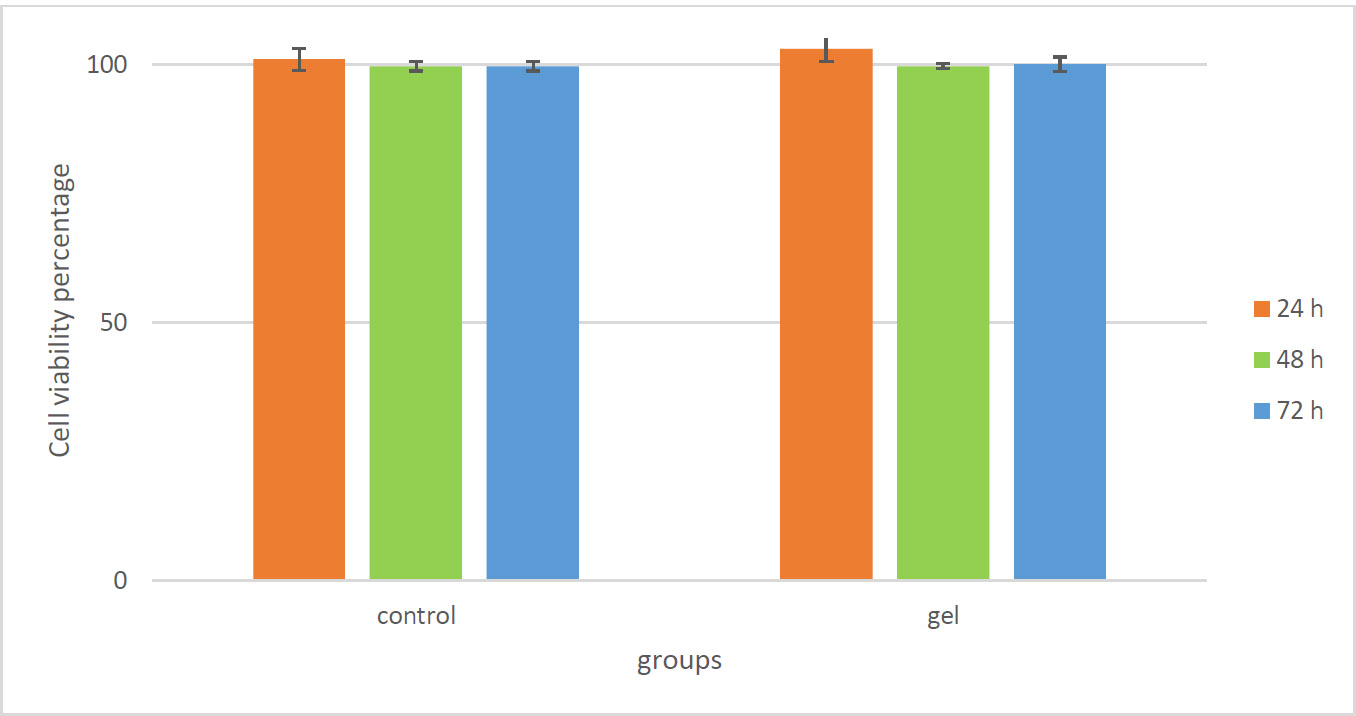
Cytotoxicity results of licorice extract gel (100 µg/mL) against HOrF cells compared to the control group at 24, 48, and 72 h.
However, another group of compounds that are present in the root of this plant are phenolic-flavonoid compounds, which have been studied in many studies for their anti-inflammatory, antimicrobial, antioxidant, cytotoxic, and anti-cancer properties [18].
The anti-inflammatory, anti-adhesive, and antimicrobial properties of licorice have beneficial effects in oral diseases such as dental caries, gingivitis, periodontitis, aphthous ulcers, and oral cancer [11]. The final goal of the study on licorice oral gel is to use its antibacterial effects or other beneficial effects in the prevention and treatment of infectious diseases of the mouth, gums, and teeth. Additionally, this gel should not be cytotoxic to the cells of the mouth, gums, and teeth. Therefore, in this study, we investigated the toxicity of this gel on HOrF cells. Despite this, the antimicrobial effects of different strains of these plants have been attributed to the presence of compounds such as terpenes and terpenoids. It has been proven that these compounds can disrupt the work of the cell membrane structures of bacteria, causing disruption in the permeability of membrane barriers, and causing the death of bacteria by disrupting the chemo-osmosis control of the cell [11]. Additionally, the phenolic compounds in licorice can disrupt the bacterial cytoplasmic membrane, increasing its permeability and releasing the main internal components, thereby destroying the bacterium. On the other hand, these active ingredients can cause dysfunction in electron transfer, ATPase enzyme activity, and absorption of nutrients by bacteria [11]. Licorice extract provides a wide range of active substances that provide essential nutrients to cells. For example, this extract contains calcium ion, glucose, potassium, vitamin C, vitamin E, fructose, zinc ion, amino acid, sucrose, and many other substances, all of which stimulate normal cell growth [19]. Licorice also contains antioxidant compounds such as flavonoids, which are reported to be 100 times more powerful antioxidants than vitamin E [20]. It also contains glycyrrhizin, which has a similar mechanism to testosterone because it is a plant hormone that increases protein production and growth [21].
In our study, the gel of this extract at a concentration of 100 µg/mL did not show cytotoxicity against HOrF cells. The study by Khazarai et al. demonstrated that licorice plant extract had a significant effect on gastric and intestinal cancer cell lines, causing the destruction of cancer cells through the induction of apoptosis, while it did not have a significant negative effect on normal cells [14]. Mohsin et al. also showed that licorice extract at a dose of 12.5 mg had no cytotoxic effect (MTT method) on normal mouse spleen cells [22]. Karimi et al. investigated the cytotoxicity of different concentrations of nanoemulsion containing licorice extract on HepG2 liver cells and SK-MEL-3 skin cancer cells by the MTT method. The results of this evaluation on HepG2 cells showed that the concentrations of 2500, 1250, and 630 μg/ml of nanoemulsion caused cell toxicity and caused the death of more than 50% of the cells (IC50=401 μg/ml) (p<0.05) while having no significant and negative effect on normal cells [23].
Recently, Heidari et al. reported that postbiotics offer a promising novel approach to dental caries prevention by targeting cariogenic bacteria and modulating the oral microbiome through multiple mechanisms. Postbiotics have revealed a multipotential capacity in preventing and managing dental caries; they can inhibit the growth of cariogenic bacteria, regulate oral microbiota, enhance host immunity, and maintain a neutral mouth pH [24].
4.1. Study Limitations
4.2. Study Strengths
4.2.1. Use of Standardized In Vitro Methods
Our study employed accepted and validated in vitro cytotoxicity protocols using Human Oral Fibroblasts (HOrF), which are relevant and commonly used cell models for evaluating oral care products. This enhances the reliability of our methodology.
CONCLUSION
Herbal medicine offers alternative ways to combat antibiotic resistance in the treatment of human oral and periodontal diseases. The aim of this in vitro study was to assess the cytotoxicity of licorice extract gel against Human Oral Fibroblasts (HOrF) for potential use in oral and dental care applications. In conclusion, licorice extract gel at 100 μg/mL demonstrated no cytotoxic effects on human oral fibroblasts, suggesting its potential as a safe ingredient in oral care formulations at the studied concentration. However, further in vivo and clinical studies are required to evaluate its therapeutic efficacy, optimal dosage, and long-term safety profile in managing oral diseases. Long-term effectiveness and toxicity tests in clinical phases and diverse populations, allowing for consideration of age, lifestyle, dietary habits, and oral health status, could establish the herbal medicine as a vital component of widespread caries prevention plans.
AUTHORS’ CONTRIBUTIONS
The authors confirm their contributions to the paper as follows: E.A.: Study conception and design, E.I.K. and H.I.K.: Data collection, draft manuscript; P.N. and M.H.S.: Analysis and interpretation of results were conducted. All authors reviewed the results and approved the final version of the manuscript.
ABBREVIATION
| HOrF | = Human Oral Fibroblas |
ETHICAL STATEMENT
The study was approved by the Ethics Committee of Tabriz University of Medical Sciences, Iran (Approval No. IR.TBZMED.VCR.REC.1402.074).
AVAILABILITY OF DATA AND MATERIALS
The data supporting the findings of the article will be available from the corresponding author [M.H.S] upon reasonable request.
FUNDING
This research was financially supported by the Vice Chancellor for Research at Tabriz University of Medical Sciences, Tabriz, Iran [Grant number: 70343].
ACKNOWLEDGEMENTS
Declared none.


