All published articles of this journal are available on ScienceDirect.
Anti-inflammatory Effects of Simvastatin Nanoparticles Coating on Titanium Healing Abutments in Dental Implants
Abstract
Introduction
In recent decades, the use of nanoparticles in dentistry, especially in implants, has attracted significant attention. Nanoparticles, due to their unique properties such as a high surface-to-volume ratio, can provide effective anti-inflammatory and antimicrobial properties that help reduce the side effects associated with implant placement. Simvastatin, an anti-inflammatory drug, has emerged as a substance with therapeutic and anti-inflammatory effects in recent studies. The aim of this study was to evaluate the anti-inflammatory effects of simvastatin-coated healing abutments compared to control and aspirin groups.
Methods
In this study, 40 titanium healing abutments coated with simvastatin nanoparticles and uncoated abutments were tested. To assess anti-inflammatory effects, dental pulp stem cells (DPSCs) were cultured in RPMI1640 medium with 10% FBS and 1% penicillin-streptomycin antibiotics. The samples (both coated and uncoated healing abutments) were placed in a sterile environment near the cells. A well was also considered for the control group (cells without any material). Cells were then stimulated with LPS (lipopolysaccharide) at a concentration of 45 µg/ml, and IL-6 and TNF-α levels were measured in all groups. Statistical analysis was performed using one-way ANOVA and Tukey's post-hoc test. A significance level was set at p≤0.05.
Results
The results revealed that the coated group showed a significant reduction in IL-6 levels (13.50±1.10) compared to the uncoated group (29.60±4.22) (p<0.0001). Similarly, the analysis of TNF-α levels showed significant reductions in the coated group (6.03±0.57) compared to the uncoated group (8.26±0.28) (p<0.0001).
Discussion
The findings of this study demonstrate the positive effect of simvastatin nanoparticles in reducing inflammation in dental implant healing abutments. These results could help improve the design of dental implants and reduce the complications arising from inflammation after surgery.
Conclusion
The use of simvastatin nanoparticles can be considered as an innovative, non-invasive therapeutic approach to improving the quality of implant treatments in dentistry.
1. INTRODUCTION
Recent advances in material science, particularly in dentistry, have introduced the use of nanoparticles as promising agents for improving the quality of dental implant treatments. These nanoparticles, due to their unique properties like high surface-to-volume ratio and biological activity, have been explored for their potential to reduce inflammation and enhance tissue repair around implants [1-4].
Dental implants are widely used to restore edentulous areas. The ability of implants to restore missing teeth when there is no bone loss around the implant fixture is of great importance, and long-term follow-ups have shown that these implants are a proven solution for replacing missing teeth [5, 6]. This treatment has become an accepted and successful treatment for completely edentulous and partially edentulous patients [7-9]. The reliability and predictability of replacing missing teeth using implants have been confirmed with an initial success rate of about 94.6% [10]. Biological changes in hard and soft tissues following tooth loss are unavoidable, and the increasing aesthetic expectations of patients have necessitated special attention to the restoration of the lost tissues with dental implants as much as possible [11]. Healing abutments are used to restore the surrounding soft tissue and create an acceptable emergence profile [12, 13]. The healing surface of dental implant abutments, which is adjacent to the gingival tissue, can cause changes in the structure of the gingival tissue. The accumulation of dental plaque in the intergingival area and healing abutments leads to increased inflammation of the soft tissue and the transfer of infection to the hard tissue. This increases the possibility of peri-implantitis and infection around the implant and threatens osseointegration, which is considered a risk for the success of the treatment [12, 13].
Adding antimicrobial materials and nanoparticles to the surface of implants can affect the tissues around the implant. For example, silver particles have a broad spectrum of effects on gram-positive and gram-negative, aerobic and anaerobic bacteria, and for this reason, they have been considered in this field [3, 13]. To reduce inflammation and improve the shape of the gingival tissue more quickly, nanoparticles can be coated on healing abutments. Nanoparticles are materials that have one or more external dimensions with a size of 1 to 100 nm. Some of the uses of nanoparticles in dentistry include composite resins, bonding systems, implant coatings, endodontic sealers, and mouthwashes [13, 14].
Statin drugs are safe and effective therapeutic agents for cardiovascular diseases. Recent studies have shown that statin drugs can be effective in improving the soft and hard tissues of the mouth [15-17]. These effects include effects on bone metabolism, anti-inflammatory and antioxidant properties, and potential effects on epithelialization and wound healing. Statins can inhibit osteoclast activity and prevent bone resorption. These substances can also accelerate epithelialization and wound healing by inhibiting the adhesion and migration of leukocytes to the site of inflammation and by reducing inflammation [18-20].
Therefore, the anti-inflammatory and osteopromotive effects of simvastatin have recently attracted the attention of researchers. This substance can accelerate bone regeneration and soft tissue healing by increasing osteoblast differentiation and stimulating angiogenesis, and reduce inflammation by inhibiting tissue-degrading enzymes such as matrix metalloproteinases (MMPs) [20, 21].
Considering the importance of maintaining the health of the soft tissue around the implant to prevent the occurrence of peri-implantitis and the transfer of inflammatory markers and microbial load to the bone around the implant and jeopardize the success of the treatment, coating abutments with anti-inflammatory compounds such as simvastatin has attracted the attention of researchers in this study. Therefore, the present study was conducted to investigate the anti-inflammatory effects of healing abutments coated with simvastatin in vitro.
2. MATERIALS AND METHODS
2.1. Study Design and Sample Size
This was an in vitro study conducted in a laboratory setting. The study design and sample size calculation were based on our previous study [22]. A total of 40 titanium healing abutments, 4.5 mm in diameter and 4 mm in height were used. A priori power analysis was conducted using G*Power software to estimate the minimum required sample size for detecting moderate effect sizes (Cohen’s f = 0.25) with a power of 80% and a significance level of 0.05. The analysis confirmed that a minimum of 10 samples per group was adequate for ANOVA-based comparisons. Therefore, the final sample size (n=40) provided statistically reliable and robust data for detecting significant differences between groups.
2.2. Titanium Healing Abutments Coating
Titanium healing abutments were coated with simvastatin nanoparticles using a standard preparation method adapted from our previous research [22]. Briefly, 40 titanium healing abutments were prepared in two groups, including the test group (titanium healing abutments coated with simvastatin nanoparticles, n=20) and the control group (titanium healing abutments without simvastatin nanoparticle coating, n=20). The dip-coating process was then applied to cover the surface of the healing abutments. The morphology and composition of coatings were evaluated by scanning electron microscopy (SEM) and X-ray diffraction analysis (XRD), respectively. The results from SEM and XRD analyses confirmed the effective deposition of gelatin-based simvastatin nanoparticles onto the abutments. Based on the Sidak test, it was observed that the average weight before coating and immediately after coating showed a significant difference. Also, the average weight between the initial time (after coating) and 1 day after coating, and 30 days after coating was not statistically significant. This indicates that the coating remained stable for at least 30 days. The difference between the initial weight (after coating) and 60 days after coating was significant, indicating that the durability of the coating decreased by day 60. The results showed that the coating of gelatinous nanoparticles containing simvastatin on the titanium healing abutments was successful, and the durability of the coating lasted for at least one month.
2.3. Anti-inflammatory Test
Dental pulp stem cells (DPSCs) were purchased from Pasteur Institute of Iran (Teharn, Iran) and cultured in RPMI1640 medium with 10% FBS and 1% penicillin-streptomycin.
Four groups were used in this study:
2.3.1. Simvastatin-coated Titanium Healing Abutment Group
Titanium healing abutments were coated with simvastatin-loaded nanoparticles, and DPSCs were seeded directly onto these coated surfaces.
2.3.2. Uncoated Titanium Healing Abutment Group
Titanium healing abutments without any coating served as the baseline control for material interaction.
2.3.3. Aspirin-treated Group (positive control)
DPSCs were cultured on uncoated titanium, and aspirin was added directly to the culture medium. Aspirin was selected as a positive control due to its known anti-inflammatory properties.
2.3.4. DPSCs without any Material as a Control Negative Group
DPSCs were seeded at a density of 1×104 cells per well on each titanium surface in 6-well plates. After 24 hours of cell attachment, inflammation was induced by adding LPS (45 μg/mL) for 24 hours. Subsequently, IL-6 and TNF-α levels in the supernatant were quantified using ELISA kits.
2.4. Statistical Analysis
Data were analyzed using one-way ANOVA, with Tukey’s post-hoc test for pairwise comparisons. The significance level was set at p < 0.05.
2.5. Ethics Approval
As we used dental pulp stem cells (DPSCs) in this study, the study started after obtaining the code of ethics from the ethics committee of Tabriz University of Medical Sciences. The ethical approval code of this project is IR.TBZMED.DENTISTRY.REC.1403.041 (Date: November 4, 2024).
3. RESULTS
3.1. IL-6 Levels
The one-way ANOVA revealed a significant difference in IL-6 levels across the experimental groups (p<0.0001). Post-hoc Tukey tests indicated that the coated group showed a significant reduction in IL-6 levels in coated group (13.50±1.10) compared to the uncoated group (29.60±4.22) (p<0.0001) (Fig. 1).
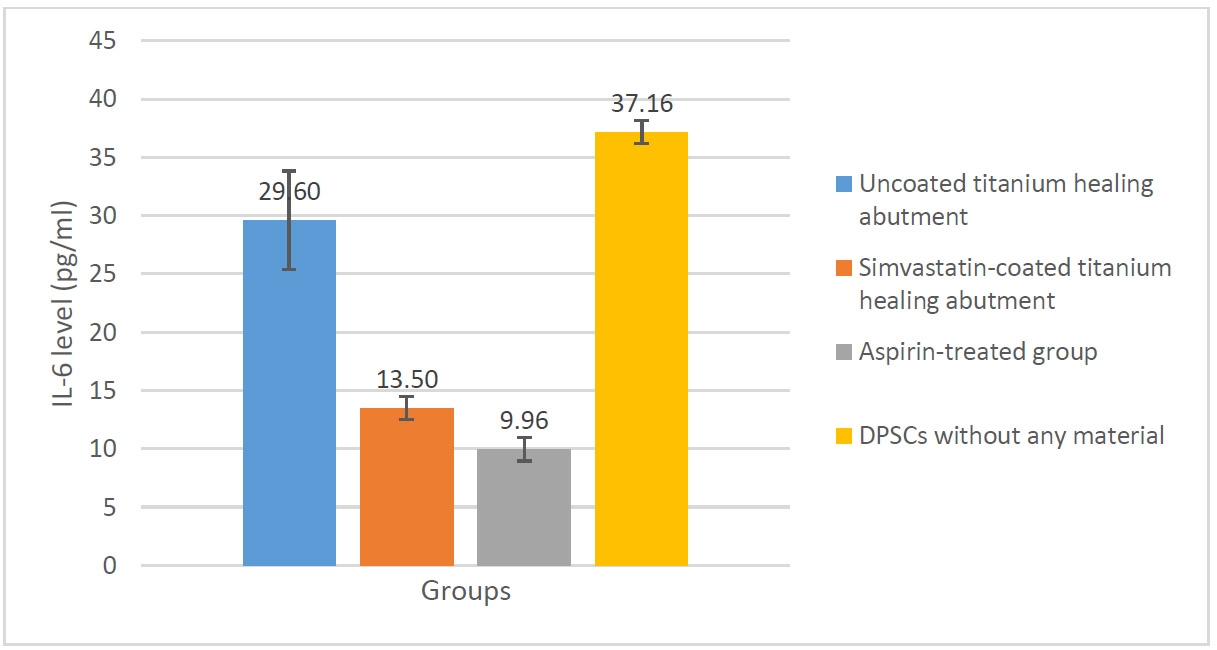
Change in IL-6 levels in the studied groups.
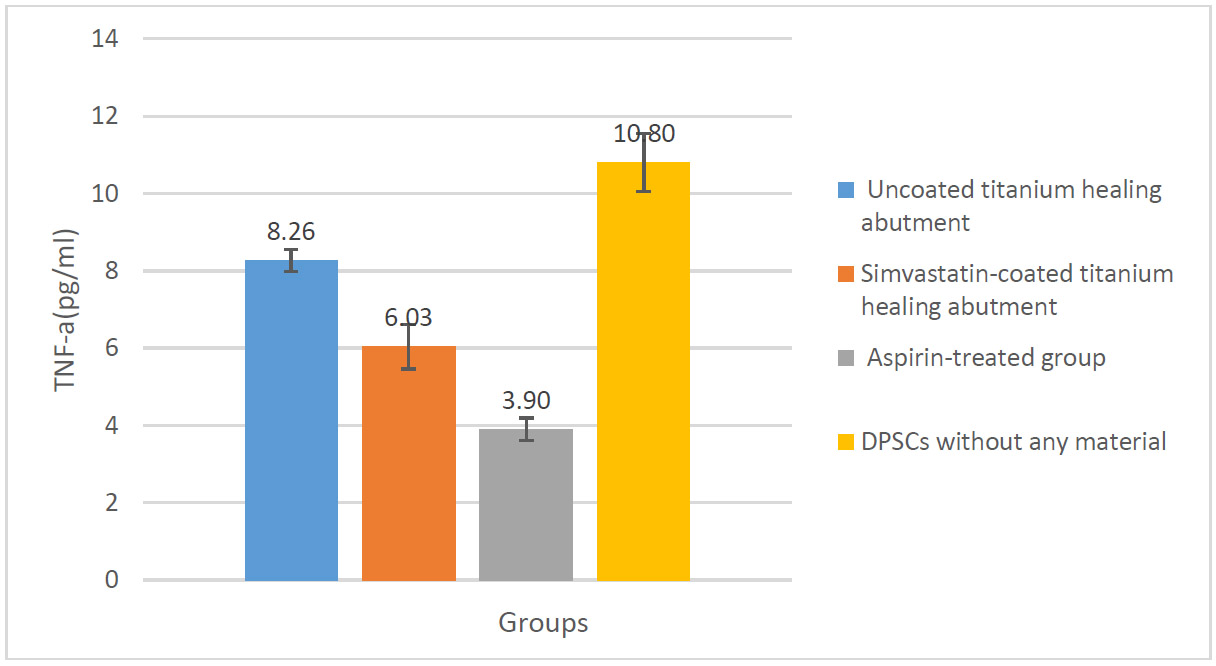
Change in TNF-α levels in the studied groups.
3.2. TNF-α Levels
The analysis of TNF-α levels showed significant reductions in the coated group (6.03±0.57) compared to the uncoated group (8.26±0.28) (p < 0.0001). The Tukey post-hoc test confirmed that in the simvastatin-coated group, TNF-α levels significantly decreased in comparison with other groups (Fig. 2).
4. DISCUSSION
The use of nanoparticles in dental implants has gained attention due to their ability to improve healing outcomes by reducing inflammation and preventing bacterial infection. In this study, simvastatin nanoparticles were applied to titanium healing abutments, with a focus on their anti-inflammatory effects. The results confirmed that simvastatin nanoparticles significantly reduced inflammatory cytokines (IL-6 and TNF-α), suggesting their potential in modulating the immune response and promoting tissue repair around dental implants.
The results of the present study showed a significant effect of healing abutments coated with simvastatin nanoparticles in reducing inflammation levels, especially in IL-6 and TNF-α indices. Specifically, in IL-6 and TNF-α indices. The results showed that coating healing abutments with simvastatin nanoparticles caused a significant decrease in IL-6 and TNF-α levels compared to the uncoated and control groups. Specifically, in IL-6 levels, the coated group showed a significant decrease, and this decrease was statistically significant compared to the control and uncoated groups (p<0.0001). Similar results were also observed in TNF-α levels, indicating the anti-inflammatory effect of simvastatin nanoparticles in reducing inflammation.
Numerous studies have shown that the use of nanoparticles can be effective in reducing inflammation caused by dental implants. For example, Dong et al. showed that the use of mesoporous silica nanoparticles (MSNs) in dental implant coatings reduced inflammation and accelerated healing [23]. This study reported results similar to the present study, particularly in reducing IL-6 and TNF-α levels in response to bacterial stimulation. The use of nanoparticles in both studies showed that these particles can effectively help reduce inflammatory responses. In contrast, Yadav et al. reported contradictory findings regarding the effects of nanoparticles on reducing inflammation after dental implant placement. In that study, nanoparticles did not show the expected anti-inflammatory effects, particularly in coated implants. In some cases, a slight increase in cytokine levels was even observed [24]. The differences in findings may be due to variations in nanoparticle composition, as well as environmental and sample-related factors.
Soares et al. investigated the anti-inflammatory, odontogenic, and proangiogenic effects of incorporating polymer scaffolds containing simvastatin on dental pulp cells (DPCs). Their results showed that the combination of low doses of simvastatin and scaffolds is a promising strategy for dentin regeneration with inflamed dental pulp tissue, by minimizing the inflammatory response and enhancing the regenerative potential of resident stem cells. According to these researchers, simvastatin, which is known as a lipid-lowering agent, suppresses the expression of inflammatory mediators, regulates odontoblastic markers, and exerts an antiangiogenic effect on endothelial cells, leading to increased vascularization and regeneration of mineralized dentin tissue [20].
The positive effect of simvastatin-coated healing abutments on IL-6 and TNF-α levels in our study may be due to the anti-inflammatory properties of simvastatin, which can have significant biochemical and cellular effects on reducing inflammation. Simvastatin nanoparticles may inhibit inflammatory proteins such as IL-6 and TNF-α due to their specific properties, including enhanced cellular penetration and direct modulation of intracellular pathways [25, 26]. Tulbah et al. investigated the anti-inflammatory and antioxidant effects of simvastatin nanoparticles on lipopolysaccharide-stimulated alveolar macrophage (AMs) NR8383 cells. Their results showed that simvastatin nanoparticles exhibited a potent inhibitory effect on nitric oxide production and cytokine secretion, without compromising cell viability. The enhanced anti-inflammatory activity of simvastatin in nanoparticulate form was attributed to its improved physicochemical stability and greater cellular uptake compared to the conventional form [25].
Okamoto et al, investigated the potential effects of statins, specifically simvastatin, on the differentiation of human dental pulp stem cells (DPSCs). They demonstrated that simvastatin at a concentration of 1 µmol/L significantly upregulated mineralization, osteocalcin, and dentin sialophosphoprotein compared to bone morphogenetic protein-2. In vivo transplantation experiments confirmed enhanced mineralized tissue formation. These findings suggest simvastatin as a promising agent for accelerating DPSC differentiation and promoting regenerative dentistry applications [27]. Weiqian Jia et al, explored the potential of simvastatin in enhancing dental pulp stem cell (DPSC) activity and its role in pulp regeneration after pulpotomy. They reported that simvastatin at 1 mmol/L significantly enhanced mineralization and mineral nodule formation. In vivo, DPSCs pretreated with simvastatin formed significantly more mineralized tissue in immunodeficient mice. Experiments in immature beagle premolars also confirmed simvastatin’s regenerative potential [16].
Both studies by Okamoto and Weiqian Jia underscore the multifaceted effects of simvastatin on DPSCs, including its role in promoting differentiation, mineralization, and pulp regeneration. While those studies emphasized regenerative aspects, their findings complement the current study’s focus on anti-inflammatory effects, supporting simvastatin’s dual therapeutic potential in dental applications.
5. LIMITATIONS
Although the results are promising, this study was limited by a small sample size and the absence of in vivo testing. Future research using larger samples and animal models would yield more robust evidence to support these findings. Additionally, the cytotoxicity of the coated implants should be tested. It is also recommended to apply more molecular techniques, such as Western blot and PCR, to assess IL-6 and TNF-α expression levels at the gene and protein levels.
CONCLUSION
This study provides strong evidence that simvastatin nanoparticles can effectively reduce inflammatory cytokines, such as IL-6 and TNF-α, in the context of dental implants. These findings suggest that simvastatin nanoparticles could be used as a promising coating material to improve the healing process of dental implants by reducing post-surgical inflammation. Further studies should include clinical trials to validate the effectiveness of simvastatin-loaded nanoparticles in human subjects. Moreover, exploring the effects of other nanoparticle-based coatings, as well as examining different cytokines and growth factors, could provide more comprehensive data on the anti-inflammatory mechanisms at play.
AUTHORS’ CONTRIBUTIONS
The authors confirm their contribution to the paper as follows: M.D.: Data curation; B.K.: Data collection; A.T.: Visualization; A.M.: Investigation; M.K.: Draft manuscript. All authors reviewed the results and approved the final version of the manuscript.
LIST OF ABBREVIATIONS
| DPSCs | = Dental Pulp Stem Cells |
| LPS | = Lipopolysaccharide |
| MMPs | = Matrix Metalloproteinases |
| SEM | = Scanning Electron Microscopy |
| XRD | = X-ray diffraction analysis |
| AMs | = Alveolar Macrophage |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The ethical approval code of this project is IR.TBZMED.DENTISTRY.REC.1403.041 (Date: November 4, 2024).
HUMAN AND ANIMAL RIGHTS
All procedures performed in studies involving human participants were in accordance with the ethical standards of institutional and/or research committee and with the 1975 Declaration of Helsinki, as revised in 2013.
AVAILABILITY OF DATA AND MATERIALS
The data supporting the findings of the article is available in the [Knowledge Repository Central, Tabriz University of Medical Sciences] at [https://dspace.tbzmed.ac.ir], reference number [73463].
FUNDING
This study, with grant number 73463 and funder ID TBZMED, has been funded by the Vice-Chancellor for Research of Tabriz University of Medical Sciences.
ACKNOWLEDGEMENTS
The authors thank the Vice-Chancellor for Research of Tabriz University of Medical Sciences for their funding support.


