All published articles of this journal are available on ScienceDirect.
Salivary Parameters Associated with Patients with Noncarious Cervical Lesions from Unstimulated and Stimulated Saliva
Abstract
Introduction
The etiology of noncarious cervical lesions (NCCLs) is based on stress, friction, and biocorrosion. Saliva is a modifying factor, related to the variability of salivary parameters and chemical composition. The aim of this study was to analyze salivary parameters in patients with NCCL using different collection methods and to identify possible biomarkers associated with NCCL.
Methods
Saliva samples were collected from subjects diagnosed with NCCL (n=20) and subjects without NCCL (n=20). Two unstimulated (spit and Salivette) and one stimulated (parafilm) method of saliva collection were used. For each collection, the salivary flow, pH, and buffering capacity were recorded. The salivary total protein and alpha-amylase concentrations were analyzed by Bradford and Western blotting methods, respectively.
Results
Regardless of the presence of NCCL, stimulated collection promoted higher salivary flow (p<0.001) and total protein concentration (p<0.001). Salivary pH showed a positive correlation with salivary flow (r=0.244; p<0.001) and buffering capacity (r=0.354; p<0.001). Individuals with NCCL had a higher concentration of alpha-amylase (p=0.013).
Discussion
The stimulation in the collection methods resulted in increased salivary flow and total protein concentration. A positive correlation was observed between pH and salivary flow, as well as between buffering capacity and salivary flow, irrespective of the presence of NCCLs. Furthermore, individuals with NCCL exhibited a higher concentration of alpha-amylase when compared with the control group.
Conclusion
Salivary parameters are directly influenced by the method of saliva collection. Therefore, standardization of saliva collection is essential, and salivary alpha-amylase is a possible biomarker for NCCL.
1. INTRODUCTION
Noncarious cervical lesions (NCCLs) are characterized by loss of dental structure at the cementoenamel junction (CEJ) in the absence of caries. NCCLs are common in dental practice and have been reported in up to 61.9% of some populations [1]. Their presence is homogeneously distributed between the two sexes, with their prevalence and levels of severity increasing with advancing age, most commonly in premolars. Despite the high prevalence of NCCL, there is still a lack of consistent diagnostic protocols in the literature regarding the etiologic factors of cervical tooth wear [2]. The origin and development of these lesions are based on three main etiological factors: stress (parafunction and traumatic occlusion) [3], friction (from toothbrush and dentifrice wear) [4], and biocorrosion (induced by endogenous and exogenous acids) [5, 6]. The combination of these factors accelerates tooth wear.
Biocorrosion is the process of tooth surface erosion, which includes all forms of chemical, biochemical, and electrochemical degradation, such as proteolytic enzymes and piezoelectric effects acting on the organic dentin matrix [7, 8]. The pathogenesis of biocorrosive processes is related to biological factors (salivary flow, pH, buffering capacity, composition, and thickness of the acquired pellicle) [9] and behavioral factors (more acidic diet, oral hygiene performed with excessive force, occupation/lifestyle, and regular exercise that allows contact with exogenous acids) [10].
In terms of biological factors, saliva is a fluid composed of approximately 99% water with a density between 1002 and 1012 g/L and a pH that may vary from 5.3 to 7.8, depending on the salivary flow [11]. This oral fluid is composed of various molecules filtered, transformed, and derived from the bloodstream, including proteins, hormones, peptides, electrolytes, mucus, various enzymes, and antimicrobial substances [12]. Saliva is responsible for numerous biological functions, such as the initial digestion of food and the maintenance of oral health through the formation of a film composed of mucins, glycoproteins, and various enzymes [13]. Saliva is considered a modifying factor in the etiology of biocorrosion-related NCCL due to the variability in its salivary parameters and chemical composition, as well as the alteration of acquired salivary pellicle formation [14]. By analyzing the composition of saliva, it is possible to identify biomarkers that indicate the presence of a certain variation in normality or disease, such as the predominance of the biological corrosive process, as well as in other oral and systemic conditions [15-18].
Compared to salivary parameters, salivary flow rate is significantly reduced in subjects diagnosed with factors related to biocorrosion [19, 20]. In these subjects, the pH is also reduced, which could lead to the activation of enzymes with the potential to degrade the tooth structure, such as metalloproteinases that promote the degradation of dentin collagen [21]. Increased oral acidity leads to a decrease in buffering capacity, which affects the saliva's ability to neutralize acidic substances [22]. For salivary analysis, saliva can be collected by the unstimulated and stimulated methods [23]. Both methods have specific advantages and limitations, such as ease of use, good adaptation, and a short and standardized collection [24-26].
The main advantage of saliva fluid collection is that it is a simple, safe, easy-to-use, and non-invasive technique. Saliva collection methods are reproducible and have lower variability. In addition, the volume of body fluid required to monitor systemic and oral changes is lower in saliva than in blood [27]. All of these advantages have contributed to the development of proteomic studies and to the use of saliva as a diagnostic tool. The determination of salivary parameters and variability in salivary composition is important for its application as a biological fluid and as a medium for identifying biomarkers in the diagnosis and prognosis of abnormal situations [28]. Therefore, the aim of this study was to analyze salivary flow, pH, and buffering capacity, identify the presence of possible biomarkers related to cervical tooth wear in subjects with NCCL, and understand the effect of different collection methods on salivary parameters and composition. The null hypothesis was that the saliva from subjects with and without NCCL would not differ in salivary parameters and composition when different collection methods were used.
2. MATERIALS AND METHODS
2.1. Saliva Collection
Saliva samples were collected, after approval by the Ethical Committee of the Federal University of Uberlandia (#942 175), from subjects diagnosed with NCCL (n=20) and from a control group without clinical signs of cervical wear (control group, n=20), comprising both genders and individuals aged 20 to 51 years. According to the inclusion and exclusion criteria, drug users (smokers, alcoholics, users of illegal substances), subjects undergoing chemotherapy and radiotherapy, subjects with xerostomia, subjects affected by sialorrhea, and individuals with syndromes and conditions involving the salivary glands were excluded from this study. The others were included in the study according to the groups. Saliva was collected between 08 a.m. and 10 a.m. The subjects signed an informed consent and were instructed to abstain from eating, drinking, and brushing their teeth for a minimum of one hour prior to the collection of their saliva. The participants were seated on comfortable chairs and remained in a rested position for fifteen minutes to control physiological stress stimulus. Subsequently, the subjects rinsed their mouths with distilled water, thereby eliminating cellular debris.
Three methods of saliva collection were adopted: spit (unstimulated), Salivette (unstimulated), and parafilm (stimulated). For the spit saliva collection, the subject was instructed to spit into a graduated Falcon tube until the desired volume of 2 mL was obtained. The duration of this procedure was meticulously recorded. The Salivette collection method entailed the utilization of a specialized high-absorption cotton-filled plastic tube (Salimetrics, State College, USA, PA). This absorption cotton was placed in the subject’s oral cavity, while the subject was seated on a chair and instructed not to chew or make exaggerated movements. After 7 minutes, the Salivette was extracted from the oral cavity and placed into a plastic tube. In the parafilm collection method, subjects were instructed to chew a specific piece of film (American National, Chicago, Ca, IL) for a period of two minutes, alternating the chewing side every 15 seconds. Saliva was collected in a plastic tube with a mean volume of 2 mL.
2.2. Salivary Parameters Analysis
2.2.1. Salivary Flow Rate
The salivary flow rate was calculated by subtracting the weight of the saliva collection tubes before and after collection using a precision electronic balance (model FA2104N, Bioprecisa, Curitiba, PR, Brazil) and then dividing by the time (in minutes) required to perform each collection.
2.2.2. Salivary pH
The collected saliva was subsequently subjected to pH evaluation using a digital pH meter (Gehaka PG1800 - São Paulo, SP, Brazil). The electrode was inserted into the sample tubes subsequent to the initial daily calibration with buffer solutions of pH 7.0 and 4.0.
2.2.3. Buffering Capacity
The saliva samples collected were submitted to buffering capacity evaluations using indicator kits (DentoBuff, Inodon, Porto Alegre, RS, Brazil). The estimation of the buffering capacity was executed by means of collecting unstimulated saliva (spit), which was obtained according to the manufacturer's instructions. In a glass receptacle, 1mL of saliva was mixed with an acid solution. Subsequently, four drops of indicator were added and vortexed to homogenize the fluids (saliva, acid solution, and indicator). After a period of five to ten minutes, carbon dioxide was extracted from the glass receptacle. The resulting color was evaluated and compared with the manufacturer's color scale, which indicated the respective pH value.
2.3. Biochemical Analysis
Saliva samples were stored at a temperature of 4°C and centrifuged at 3000 xg for 15 minutes. The pellets were discarded, and aliquots of the supernatants were stored at -80ºC for subsequent evaluation.
2.3.1. Total protein Concentration (Bradford protocol)
The dosage of total proteins in saliva samples was determined using the Bradford protocol (1976) with adaptations made for use with a microplate format and bovine serum albumin serving as a standard [29]. In each microplate well, 5 µL of saliva, 95 µL of deionized water, and 200 µL Bradford reagent were added. The assay was performed in duplicate, and the readout was taken at 595 nm at room temperature using a VersaMax (Molecular Devices, Sunnyvale, California, USA).
2.3.2. Biomarker Verification - Alpha-amylase Concentration (Western blot)
Saliva samples were denatured under reducing conditions and applied to polyacrylamide gels (SDS-PAGE) with a 5-22% gradient [30]. The electrophoretic separation of proteins was carried out with a constant current of 35mA. The gels were subjected to staining with Coomassie Brilliant Blue R-250 and subsequently scanned. The remaining samples were used for Western blotting, a technique based on the interaction between an antibody and an antigen. The gels previously used in one-dimensional electrophoresis were subjected to the immunoblotting technique. In this experiment, saliva samples were electro-transferred to a nitrocellulose membrane (Millipore, Bedford, MA, USA) for 2 hours under a constant current of 100 mA [31]. The membranes were then stained with 0.5% Ponceau and blocked with 5% nonfat milk in PBS-Tween for a period of 4 hours. Subsequently, the membranes were incubated with their respective primary antibody, alpha-amylase antibody, according to Da Silva Santos [32], for 12 hours. Subsequently, these membranes were incubated with secondary antibody, horseradish peroxidase-conjugated anti-rabbit (Santa Cruz Biotechnology, Santa Cruz, CA, USA), for 4 hours. The use of PBS-Tween buffer (0.05%) was employed to wash the membranes between the incubation procedures [33]. The membranes were incorporated into a solution that contained the substrate for the enzyme to which the secondary antibody was conjugated for detection by chemiluminescence. This detection process was facilitated by the ECL system microplate reader (Molecular Devices Corporation, CA., USA).
2.4. Statistical Analysis
The data was analyzed using SigmaPlot software 12.0 (Systat Software Inc., San Jose, California, USA) at a 95% confidence level. Two-way repeated measures analysis of variance and the Tukey Test were employed to determine the mean salivary flow and protein concentration values. Kruskal-Wallis and Tukey Tests were used for pH, and Mann-Whitney was employed for buffering capacity and alpha-amylase concentration. The Pearson correlation coefficient was calculated for the following pair of variables: pH and salivary flow, buffering capacity and salivary flow, pH and buffering capacity, and alpha-amylase concentration and protein concentration.
3. RESULTS
3.1. Salivary Parameter Analysis
The salivary flow, pH, and buffering capacity of subjects with and without NCCL are described in Figs. (1-3), respectively. The correlation between the salivary parameters was shown in Fig. (4). The salivary flow exhibited no discernible influence from the presence of NCCL, irrespective of the method of collection (p=0.780). Nevertheless, this parameter was affected by the salivary collection method (p<0.001).
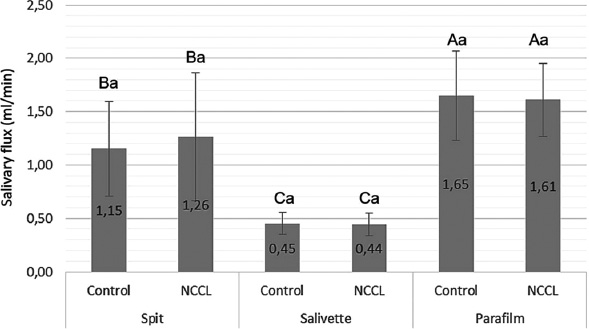
Salivary flow mean (mL/s) and standard deviation for comparison of salivary collection methods, and the presence of NCCL. Capital letters for comparisons of salivary collection methods; and Lowercase letters for comparisons of subjects with and without NCCL. (Two-way repeated measures analysis of variance and Tukey Test; p<0.05).
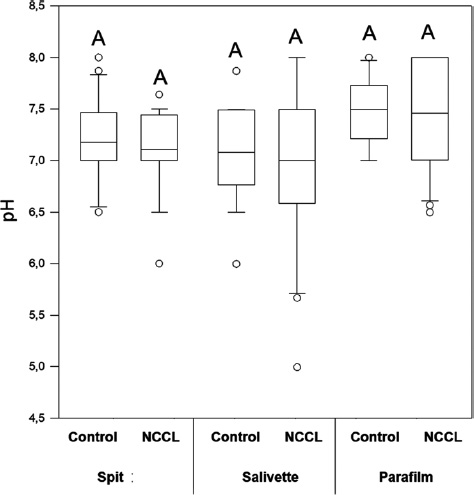
pH median comparing salivary collection methods and the presence of NCCL. Capital letters for comparisons for all groups. (Kruskal-Wallis and Tukey Test; p<0.05).
The parafilm-stimulated collection showed a mean flow rate of 1.63mL/min, followed by unstimulated spit (1.20mL/min) and salivette (0.44mL/min) (Fig. 1). The pH parameter exhibited comparable values for all groups (Fig. 2). Similarly, analogous to the phenomenon of pH, the buffering capacity showed similar conditions for subjects with and without NCCL for the unstimulated spit salivary collection method (p=0.510) (Fig. 3). The correlation between salivary flow and pH was observed (correlation coefficient = 0.244; p<0.001) (Fig. 4A), and no correlation was observed between the salivary flow and the buffering capacity (p=0.990) (Fig. 4B). The investigation revealed a positive correlation between pH and buffering capacity (correlation coefficient = 0.354; p<0.001) (Fig. 4C). The investigation revealed no association between protein concentration and alpha-amylase concentration (p=0.490) (Fig. 4D).
3.2. Biochemical Analysis
The protein concentration (Bradford), salivary protein, and alpha-amylase concentrations (Western Blot) of subjects with and without NCCL are described in Figs. (5-8). The correlation between the biochemical analyses is described in Fig. (4). The protein concentration was found to be unaffected by the presence of NCCL, regardless of the collection method (p=0.474). However, the salivary collection method was found to have a significant impact on the resultant parameter (p<0.001). The mean protein concentration of saliva collected using the Salivette collection method was found to be lower (5.91 mg/mL) compared with the spit (20.67 mg/mL) and parafilm collection methods (21.76 mg/mL) (Fig. 5). However, a visual evaluation of the salivary protein profile, as represented by the relative mass (Mr), revealed the presence of more visible polypeptides in the group with NCCL compared to the group without NCCL, mainly in the band that represented the molecular weight of the alpha-amylase marker (52kDa) [25], as demonstrated in SDS-PAGE (Fig. 6). An evaluation of the salivary alpha-amylase concentration using different methods of saliva collection revealed that subjects with NCCL exhibited higher median alpha-amylase concentration values (19319.18 OD) compared to those in the control group (14839.00 OD) (p=0.013) (Fig. 7), which was corroborated by Western Blot (Fig. 8) and pixel density values.
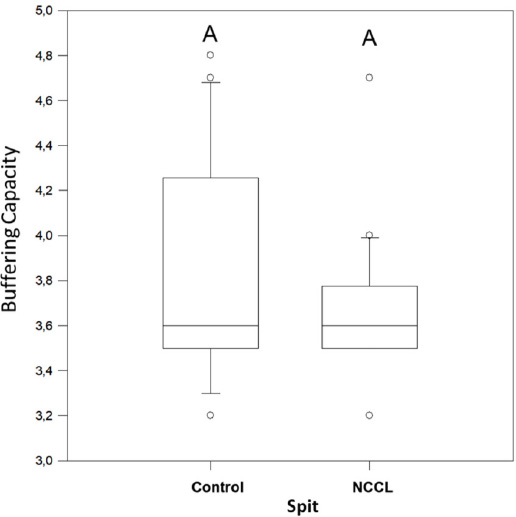
Buffering capacity median for spit salivary collection method comparing subjects with and without NCCL. (Mann-Whitney; p<0.05).
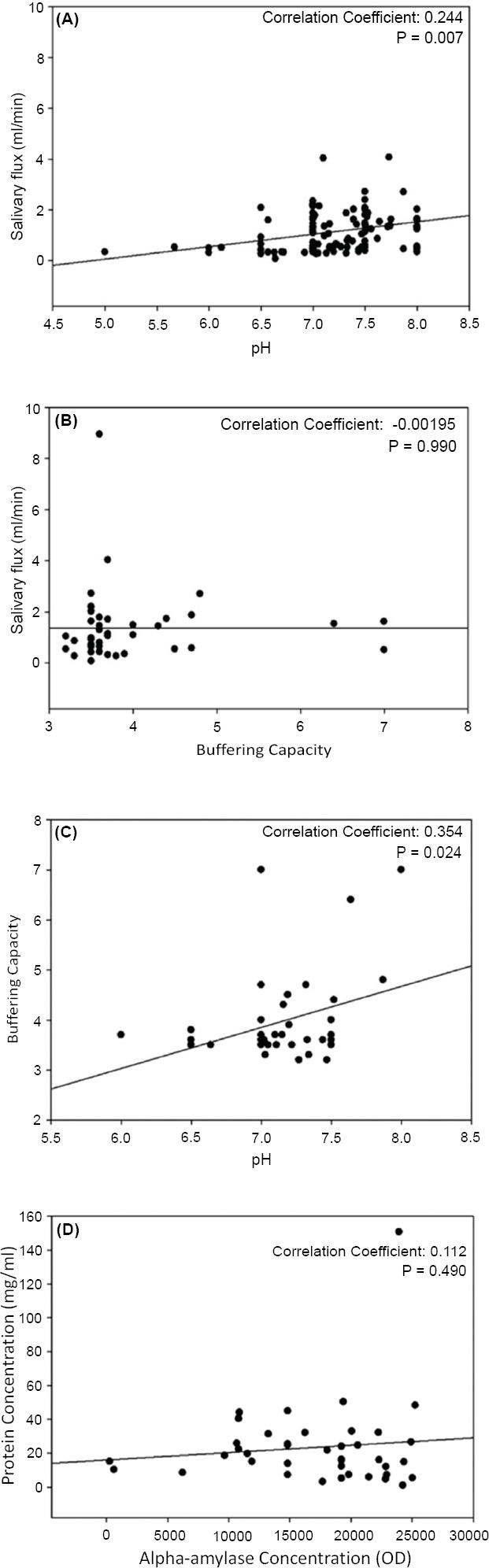
Pearson analysis (p<0.05) for correlation between pH and salivary flow (mL/s) (A), buffering capacity and salivary flow (mL/s) (B), pH and buffering capacity (C) and alpha-amylase concentration (U/mL) and protein concentration (U/mL) (D).
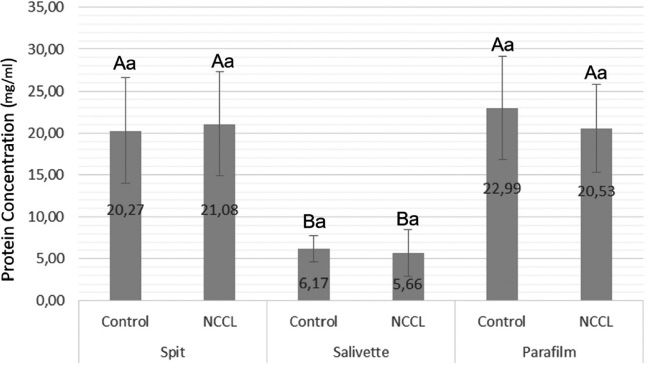
Protein Concentration mean (U/mL) and standard deviation comparing salivary collection methods and the presence of NCCL. Capital letters for comparisons of salivary collection methods; and Lowercase letters for comparisons of subjects with and without NCCL. (Two-way repeated measures analysis of variance and Tukey Test; p<0.05).
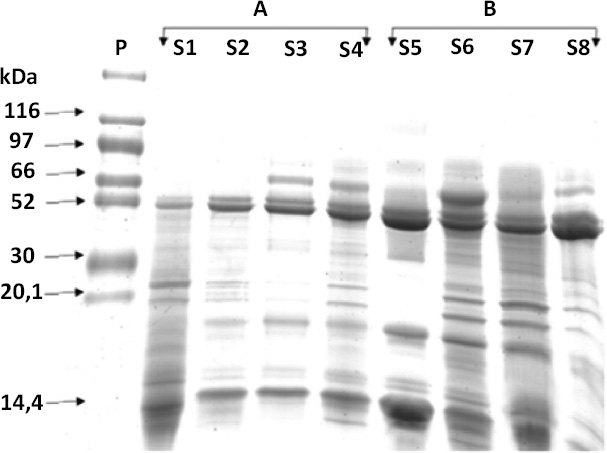
Salivary protein profile represented by relative mass (Mr), comparing the presence of polypeptides visible in saliva samples of the individuals without NCCL (A) and with NCCL (B), in SDS-PAGE.
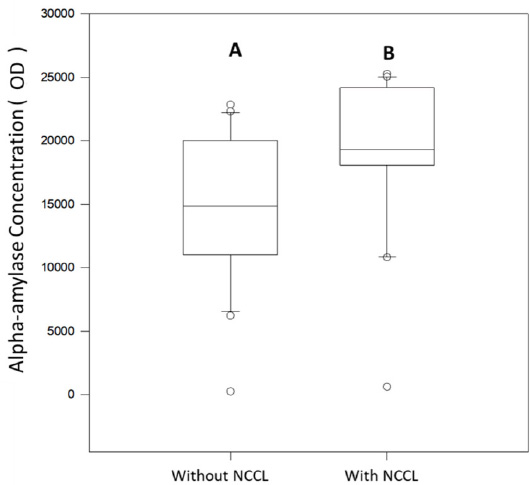
Alpha-amylase concentration (U/mL) median for spit salivary collection method comparing subjects with and without NCCL. (Mann-Whitney; p<0.05).
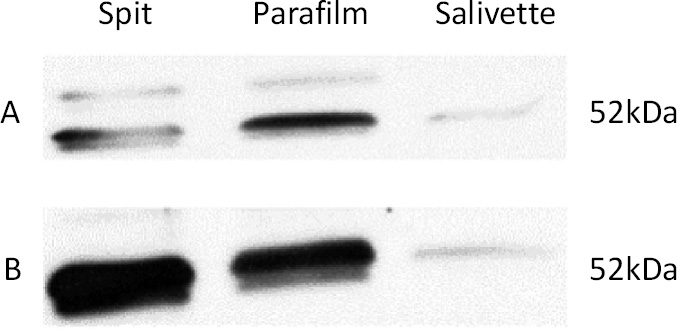
Alpha-amylase concentration on different methods of salivary collection in control group (A) and in subjects with NCCL (B), by Western Blot.
4. DISCUSSION
To the best of our knowledge, this is the first study to evaluate salivary biomarkers for noncarious cervical lesions. Concerning the collection methods, it was demonstrated that stimulation resulted in increased salivary flow and total protein concentration. Furthermore, a positive correlation was observed between pH and salivary flow, as well as between buffering capacity and salivary flow, irrespective of the presence of non-carious cervical lesions (NCCLs).
Salivary secretion is a process that is mediated by a combined parasympathetic and sympathetic stimulus. The adrenergic system is primarily responsible for the secretion of water and proteins, while the cholinergic system plays a crucial role in regulating the secretion of electrolytes [34]. The proteins and electrolytes in the saliva can serve as biomarkers, indicating potential oral or systemic alterations. Biomarkers are substances that have the capacity to monitor states of abnormality and are indicative of certain conditions [35]. The aim of this study was to evaluate salivary parameters in patients with noncarious cervical lesions using different collection methods and determine whether alpha-amylase enzyme could be a biomarker of NCCL, since this protein is known to be a stress indicator and its release is regulated by the sympathetic nervous system [36].
The authors observed that individuals with NCCL exhibited a higher concentration of alpha-amylase when compared with the control group. Increases in amylase concentration may be one of the several actions involved in activating the body’s resources to cope with stressful events or threats to homeostasis. Therefore, the monitoring of this salivary biomarker may contribute to the elucidation of the profile of patients with cervical tooth wear. In this regard, research has demonstrated a substantial correlation between alpha-amylase and catecholamines (r=0.64 for norepinephrine and r=0.49 for epinephrine) in physiological stress [37].
Saliva is a suitable diagnostic fluid and is considered the primary defense mechanism against the presence of abnormalities [38-40]. In an acidic environment, salivary flow promotes pH neutralization through the buffering capacity. Furthermore, elevated levels of salivary flow contribute to attenuating the consequences of higher concentrations of acid in the oral environment [41]. Consequently, substantial correlations were identified among salivary flow, pH, buffering capacity, and salivary composition, which played a significant role in the diagnosis of systemic and oral diseases, including NCCL [42].
Although the literature suggests a correlation between reduced salivary flow and an elevated risk of NCCL onset [14], the present study found no such association. The salivary flow measurements were comparable among subjects with and without NCCL. This result could be explained by the physiological phenomenon of reflex hypersalivation in subjects diagnosed with endogenous biocorrosive processes, classified as “esophageal-salivary reflex”. [15] Salivary stimulation and the physiological phenomenon that results in increased salivary flow into the oral environment with an acidic pH have been demonstrated [20]. Furthermore, the volume of saliva secreted is strongly influenced by the density of the saliva, and the presence of higher volumes of viscous saliva reduces the salivary flow.
Although the presence of NCCL had no influence on the salivary flow, the methods of collection showed different results. The parafilm-stimulated collection showed a higher mean flow rate value. The results were in agreement with those of a recent study [43], which also demonstrated an increase in salivary flow promoted by parafilm-stimulated collection. This phenomenon is attributed to the combined effects of mechanical and gustatory stimulation on the salivary glands, resulting in the activation of autonomic receptors that are responsible for the secretion of saliva and the control of its flow. Moreover, the nervous fibers implicated in both parasympathetic and sympathetic salivary secretions are predominantly activated by chewing [44].
According to the multifactorial etiology of NCCL, biocorrosion and saliva are considered important factors in the origin and development of cervical wear [14]. Biocorrosion is defined as the process of wear of dental hard tissue demineralized by acidic substances [45]. The presence of endogenous acids was attributed to gastroesophageal reflux disease, which causes the retrograde movement of gastric juices into the esophagus. Other factors related to the biocorrosion process and changes in salivary parameters were dietary and occupational habits [46]. When exposed dentin is stimulated by external factors such as chemical, thermal, tactile, and osmotic pressure, it triggers a painful symptomatology known as dentin hypersensitivity [47]. However, this response was not reflected in salivary pH parameters.
The salivary pH value remained consistent among subjects, irrespective of the presence of NCCL and the collection method. It could occur because the pH variation is not determined only by the presence of H+ ion but also by the interactions between the components of a solution present in the oral environment, such as concentrations of calcium and phosphate in the solution [48]. Therefore, the disparities in salivary composition with regard to organic and inorganic substances elicit divergent protective mechanisms and repair capacity in individuals [14]. Moreover, the quantity of H+ ions, salivary composition, and pH are also influenced by the subject’s age, the chronic action of acid challenge, the site in the oral cavity, and the time of saliva collection [46].
The buffering capacity is responsible for neutralizing and clearing the acid that causes tooth wear [21], thereby contributing to the maintenance of an oral pH level that remains constant and reduces the corrosive action of the acids and the loss of dental structure [47]. The buffering capacity and pH values exhibit a positive correlation for the unstimulated spit salivary collection method. As the pH values increase, the buffering capacity also increases. This positive correlation may promote a similar oral acidity in both the subjects with and without NCCL.
Moreover, different dietary patterns potentiate the salivary buffering capacity, thereby promoting a neutral pH environment and reinforcing the impact of dietary habits on the prevalence of dental corrosion, irrespective of the presence of NCCL [49]. Moreover, the buffering capacity of saliva is less effective during periods of low flow, such as in unstimulated saliva. In this situation, the concentration of phosphate present in saliva is analogous to that in high-flow saliva, with the latter being the predominant factor contributing to the acid-neutralizing capacity [50]. In the present study, the buffering capacity was determined using the unstimulated spit salivary collection method exclusively [15], according to the manufacturer’s guidelines for the dental buff (DentoBuff, Inodon, Porto Alegre, Brazil).
Although the salivary total protein concentration remained consistent, irrespective of the presence of NCCL, this parameter was affected by the salivary collection method. The samples obtained by the Salivette collection method demonstrated lower mean protein concentration in comparison with the other collection methods. This may be attributed to the device’s poor recovery and absorption capacity for certain protein compounds [51]. Furthermore, variations in protein excretion rates may be observed due to variations in the volume of saliva prior to collection or the absence of pre-concentration. This statement was supported by the unique profile exhibited by the Salivette collection method, which demonstrated a higher concentration of organic and inorganic compounds in the stimulated saliva compared to the non-stimulated collection methods [52, 53].
In addition to the differences observed in the saliva parameters according to different collection methods, the study’s data is constrained to a single timeframe collection. While the flow rate of saliva and the secretory levels of salivary components are known to be diurnal, the digestive system's circadian clock has been shown to regulate the levels of digestive enzymes and gastric acid, with a peak in the evening [54].
The observation that alpha-amylase concentration was higher in subjects with NCCL, irrespective of the collection method, suggests that alpha-amylase may serve as a potential biomarker for this condition. However, these observations did not extend to the salivary total protein concentration, which exhibited no significant differences between the control and NCCL patient groups. Despite the multifactorial etiology of NCCL, these findings suggest that the analysis of saliva parameters and biomarkers can be applied as an aid in diagnosing and identifying risk factors and possible causes of NCCL.
CONCLUSION
Standardizing the method of saliva collection is imperative to minimize variations in saliva composition and flow, pH, and buffering capacity among individuals. Although no differences in salivary parameters were observed among groups, the higher concentration of alpha-amylase in the saliva of NCCL patients implied the potential for further investigation into its role as a biomarker. This approach aims to elucidate the mechanisms through which biological and behavioral factors contribute to the process of tooth surface erosion in the NCCL etiology.
This study has limitations inherent to its cross-sectional design, such as the relatively small sample size and the influence of inherent multifactorial variables. Its strengths include the comparative analysis between stimulated and unstimulated saliva, in addition to the use of sensitive methods such as Bradford and Western blotting, which provide robustness to protein quantification. This approach of salivary biomarkers offers a relevant contribution to the understanding of the pathophysiology of NCCL.
For future investigations, the adoption of longitudinal designs with larger samples and control of confounding factors is recommended. The inclusion of other biomarkers (e.g., electrolytes, antioxidant enzymes) and the use of omics approaches may broaden the understanding of the influence of saliva on the etiopathogenesis of NCCLs.
AUTHORS’ CONTRIBUTIONS
It is hereby acknowledged that all authors have accepted responsibility for the manuscript's content and consented to its submission. They have meticulously reviewed all results and unanimously approved the final version of the manuscript.
LIST OF ABBREVIATIONS
| NCCLs | = Noncarious Cervical Lesions |
| CEJ | = Cementoenamel Junction |
| pH | = Hydrogen Potential |
| SDS-PAGE | = Sodium Dodecyl Sulfate–Polyacrylamide Gel Electrophoresis |
| PBS | = Phosphate Buffered Saline Solution |
| ECL | = Enhanced Chemiluminescence |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The approval by the Ethical Committee of the Federal University of Uberlandia, Brazil (#942 175).
HUMAN AND ANIMAL RIGHTS
All procedures performed in studies involving human participants were in accordance with the ethical standards of institutional and/or research committee and with the 1975 Declaration of Helsinki, as revised in 2013.
AVAILABILITY OF DATA AND MATERIALS
The data supporting the findings of the article will be available from the corresponding author [P.V.S] upon reasonable request.
FUNDING
This study was funded by Minas Gerais State Research Support Foundation, Funder ID: 21.949.888/0001-83, Awards/Grant number: PIBICMG2014-SAU024.
ACKNOWLEDGEMENTS
Declared none.


