All published articles of this journal are available on ScienceDirect.
Simultaneous Sinus Augmentation and Implant Placement in the Atrophic Posterior Maxilla: A Systematic Review
Abstract
Background
Rehabilitating the severely resorbed posterior maxilla presents a significant clinical challenge. Successful implant placement in the posterior maxilla requires sufficient bone volume, critically influencing the surgical approach. Limited bone height necessitates careful assessment to determine the appropriateness of a one-stage or two-stage lateral sinus lift procedure. A two-stage approach involves preliminary sinus augmentation followed by implant placement after graft maturation (typically 6 months or more).
Objective
This systematic review examines the feasibility and efficacy of a one-stage approach, which includes simultaneous maxillary sinus floor augmentation and implant placement in atrophic maxillae with less than 5 mm of residual bone height. The primary focus is on analyzing implant survival rates and providing evidence-based guidance for optimizing treatment protocols.
Methods
An electronic search was conducted in MEDLINE-PubMed, Google Scholar, and ScienceDirect, supplemented by a manual review of reference lists. This review included English-language publications on maxillary sinus floor augmentation using various biomaterials. All clinical trials meeting the inclusion criteria were considered.
Results
Nine studies met the eligibility criteria (5 prospective cohort studies, 1 case report, 2 controlled clinical trials, and 1 randomized controlled trial). A total of 801 implants were included in the analysis. Implant survival rates ranged from 90% to 100%.
Conclusion
This comprehensive analysis will contribute to a deeper understanding of factors influencing successful implant integration in the posterior maxilla, particularly regarding the potential to significantly reduce treatment time by eliminating the graft maturation period required for two-stage procedures.
1. INTRODUCTION
Insufficient bone volume in the posterior maxilla is a common anatomical limitation for implant placement. This bone loss significantly impacts the selection of the most suitable rehabilitation method for edentulous patients. While removable prostheses can serve as a treatment option for posterior edentulism, studies have shown that this approach can negatively affect masticatory function and potentially compromise the prognosis of adjacent teeth when compared to implant-supported rehabilitations [1, 2].
The placement of dental implants in the posterior maxilla poses a significant challenge due to the frequent occurrence of vertical bone height reduction. This reduction is often attributed to pneumatization of the maxillary sinus, the natural process of aging, and early tooth loss. The presence of D4 bone quality in this region further exacerbates the difficulty. To address these challenges, a range of treatment modalities are available, tailored to the specific degree of bone atrophy. These include sinus augmentation, indirect sinus lifts, short implant placement, vertical alveolar ridge regeneration, and the utilization of alternative implant sites such as the tuberosity, pterygoid process, zygoma, or placement of tilted implants [3].
Primary stability is a crucial element for the success of implants. When there is micromotion of the implant, it can reduce secondary stability and result in fibrous encapsulation rather than osseointegration. Additionally, micromotion at the bone-implant interface exceeding 150 µm has been associated with compromised biological healing [4].
Witoonkitvanich et al. investigated the stability of immediate dental implants placed in fresh molar extraction sockets, comparing those in the maxilla and mandible. Throughout the observation period, the average Implant Stability Quotient (ISQ) was above 70 for both the maxilla and mandible, suggesting that immediate loading is feasible in the molar area. Notably, the implants in the mandibular molar region demonstrated significantly higher mean ISQ values compared to those in the maxillary molar region [5].
The sinus floor elevation procedure using the standard lateral approach was developed in the late 1970s to create a suitable environment for the implant placement. This technique, initially introduced by Tatum, was later refined by Boyne and James as well as Wood and Moore [6, 7].
To restore bone volume in the atrophied posterior maxilla, a sinus lift procedure is performed by elevating the sinus membrane and using bone grafts to maintain the space, facilitating bone regeneration according to the principles of guided bone regeneration (GBR) [8].
According to the principles of guided bone regeneration, during the sinus lift procedure, the bone graft serves as a space holder beneath the elevated sinus membrane [8]. This biological insight underscores the significance of the graft material's osteoconductive properties in the sinus lift process. The osteogenic source for bone healing originates from two anatomical areas: the basal bone of the sinus cavity and the periosteum, which is the basal cell layer of the Schneiderian membrane. In line with this biological principle, Mish developed a classification for treating edentulous posterior maxilla based on the available bone beneath the antrum and the width of the ridge [9].
This classification, which assesses the ability to stabilize the implant during the initial surgery, outlines three treatment options. Clinically, a minimum native bone crest height of 3mm is required for a one-stage procedure. The choice between one- or two-stage techniques depends on the residual bone available and the potential for achieving primary stability. A one-stage technique using either a lateral or transalveolar approach is recommended for higher crests, while a two-stage technique with a lateral window approach is suggested for lower crests, with implant placement occurring after a healing period. Severely resorbed maxillae pose significant challenges for bone regeneration and implant success, as studies indicate less than 10% bone regeneration [10] and a 5–20% risk of implant failure even with autogenous bone grafts [11].
Several techniques have been developed to achieve adequate bone dimensions for implant placement in the atrophic posterior maxilla [12, 13]. Recent advancements in surgical techniques and biomaterials have led to excellent outcomes for implant-supported restorations [14-16].
Sinus floor augmentation procedures have consistently demonstrated high implant survival rates exceeding 90%, as confirmed by recent systematic reviews [17-19]. However, pre-existing sinus conditions may require ENT (ear, nose, and throat) specialist evaluation, and complications like membrane perforation, bleeding, and post-operative discomfort can occur, particularly with lateral approaches [20, 21].
Studies suggest a minimum bone height of 4-5 mm is needed for immediate implant placement in the same surgery of sinus lifting [22-25]. This is supported by Geurs et al. (2001), who found significantly higher implant loss rates when residual bone height was 4 mm or less compared to 5 mm or greater [26].
The aim of this systematic review was to assess the efficacy of a one-stage surgical procedure for implant placement in maxillary sinus floor augmentation and to examine implant survival rates, focusing on cases with an average residual bone height of 5 mm or less.
2. METHODS
This systematic review was carried out in accordance with the guidelines outlined in the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) statement [27].
2.1. Focused Question
The focused research question was:
Is maxillary sinus floor augmentation and simultaneous implant placement in the atrophic posterior maxilla (residual bone height less than 5 mm) effective in restoring lost teeth and shortening overall treatment time?
2.2. Type of Studies
The authors included all human clinical studies regarding maxillary sinus floor augmentation and simultaneous implant placement in atrophic posterior maxillae. The search utilized relevant keywords and focused on English-language publications. The selection process prioritized studies that defined implant survival rates. All study designs were considered, including prospective, retrospective, case reports, and randomized controlled trials. Abstracts, letters, and reviews were excluded.
2.3. Type of Participants
The study included all patients requiring restoration of lost teeth in an atrophic posterior maxilla with a vertical bone height of 5 mm or less.
2.4. Type of Intervention
Studies investigating one-stage maxillary sinus floor augmentation (MSFA) and simultaneous implant placement in cases of atrophic posterior maxilla with a vertical bone height of 5 mm or less were included. Studies that did not perform lateral sinus floor elevation in the posterior maxilla or did not place implants immediately were excluded.
2.5. Type of Outcome Measures
The primary outcome was the survival/success rate of the simultaneously inserted implants with maxillary sinus floor elevation in the atrophic posterior maxillae with a minimum follow-up period of four months.
The survival rate represents the stability of functioning implants, with no implant removal during follow-up. In contrast, the success rate is based on factors including soft and hard tissue levels, probing pocket depth, and bleeding upon probing.
2.6. Source of Information and Search Strategy
For the identification of studies to be involved in this review, the authors conducted a search strategy for the following electronic databases: MEDLINE-PubMed, Google Scholar, and ScienceDirect. The review included human clinical studies published from inception up to January 2025. The search strategy also involved manually reviewing the reference lists of all selected full-text articles. The search utilized relevant keywords and focused on English-language publications. The following keywords were applied: (“residual bone height” OR “atrophic maxilla” OR “atrophic posterior maxilla “AND “sinus lift “OR” sinus floor elevation” OR “maxillary sinus augmentation”) OR (“lateral window” OR “maxillary sinus grafting” OR “lateral approach” OR “sinus graft” AND “dental implants” OR “one stage sinus elevation”) OR (“maxillary sinus lift” OR “sinus lift” AND “simultaneous implant placement” OR “immediate implant placement”).
2.7. Study Selection
Two independent reviewers conducted the literature search using predefined keywords. Following an initial screening of titles and abstracts, any discrepancies were resolved through discussion. Subsequently, the full text of the selected articles was reviewed.
2.8. Data Items
Data from the articles' full texts were tabulated and compared. The following variables were evaluated: survival/success rate of the simultaneously inserted implants with lateral sinus floor elevation in atrophic posterior maxillae, the use of bone substitutes, and the duration of follow-up (Table 1).
| Author, Year/ Refs. | Study Design | Group Treatment | Number of Patients |
Number of Implants |
Survived Implants |
Failed Implants |
Grafting Material | Mean Follow-up |
Success/survival Rate |
|---|---|---|---|---|---|---|---|---|---|
| Pistilli et al. 2022 [31] | Prospective cohort study |
SFE and simultaneous Implant placement |
43 | 113 | 113 | 0 | MP3, Heterologous cortico-cancellous bone mix | 5.11year (SD: 2.47 | 100% |
| Felice et al. 2013 [28] | Randomized controlled trial | SFE and simultaneous Implant placement |
30 | 30 | 27 | 3 | Bone substitute | 4months | 90% |
| SFE and delayed Implant placement |
30 | 30 | 29 | 1 | 96.6% | ||||
| Simonpieri et al, 2011 [35] |
Prospective cohort study |
SFE and simultaneous Implant placement |
20 | 52 | 52 | 0 | L-PRF CLOTS | 72 (24-72) months |
100% |
| Canullo et al, 2012 [34] |
Prospective cohort study |
SFE and simultaneous Implant placement |
30 | 67 | 65 | 2 | HA + SILICE GEL (nanobone) |
24 months | 97,01 % |
| Manso et al, 2010 [33] |
Prospective cohort study |
1 group: Autogenous bone and Bioactive resorbable graft |
45 | 160 | 158 | 2 | Autogenenous ( retromolar)+ (syntetic bioactive resorbable graft) |
61,7 (20-132) months |
98% |
| Rodriguez et al, 2003 [32] |
Prospective cohort study |
SFE and simultaneous Implant placement |
15 | 70 | 65 | 5 | DPBB+ PRP | 6-36 months | 92.9% |
| Mendonca- Caridad et al, 2013 [29] |
Controlled clinical trial | SFE and simultaneous Implant placement |
15 | 46 | 44 | 2 | Calvaria+ PRP+B-TCP |
12,8 months | 95.7% |
| Cha et al, 2014 [11] | Controlled clinical trial | SFE and simultaneous Implant placement (RBH < 4mm) |
217 | 262 | 255 | 7 | Xenogenic bone (Bio-Oss®) | 57.1 ± 15.6 (36-98) months | 98.91% |
| SFE and simultaneous Implant placement (RBH < 4mm) |
200 | 191 | 9 | 96.54% | |||||
| Sleman et al, 2025 [30] | Case report | SFE and simultaneous Implant placement |
1 | 1 | 1 | 0 | Autogenous bone ring + allograft bone substitute | 12 months | 100% |
2.9. Data Selection Process
Following PRISMA guidelines, reviewers extracted and tabulated data from the selected articles, resolving any disagreements by consensus.
2.10. Quality Assessment
The methodological quality of all included studies was independently evaluated in duplicate by two reviewers. The Cochrane Collaboration's tool for assessing the risk of bias was used for RCTs and CCTs. The following criteria were considered: sample size determination; randomization sequence (selection bias); allocation concealment (selection bias); blinding of operators and participants (performance bias); incomplete outcome data (attrition bias); selective outcome reporting (reporting bias); group imbalance; and follow-up duration. Each domain's risk of bias was judged as “high risk” or “low risk” based on the extracted information. Table 2 summarizes the results of this quality assessment. Prospective studies were analyzed using the Newcastle-Ottawa Scale (NOS), as shown in Table 3.
| - | - | Felice et al 2013 [28] | Cha et al [11] |
|---|---|---|---|
| Random sequence determination (selection bias) | A | Low risk | High risk |
| - | B | A computer-generated restricted random list was created | No randomization: group 1 included patient with RABH <5mm; group 2 included patients with RABH >5mm |
| Allocation concealment (selection bias) |
A | Low risk | High risk |
| - | B | After flap elevation, the opaque sealed envelope containing the group allocation code, which was sequentially numbered, was opened | - |
| Blinding of participant and researchers |
A | High risk | High risk |
| - | B | Both the patient and the surgeon were aware of the allocated arm and would know the randomized type of treatment performed | Both the patient and the surgeon were aware of the allocated arm |
| Blinding of outcome assessment |
A | Low risk | High risk |
| - | B | One dentist, who was not involved in the treatment of the patients, made all clinical assessments without knowing the group allocation; therefore, the outcome assessor was blinded | Not reported |
| Incomplete outcome data (attrition bias) |
A | Low risk | Low risk |
| - | B | Losses to follow up were reported and specified |
Losses to follow up were reported and explained |
| Selective reporting (reporting bias) |
A | Low risk | Low risk |
| - | B | All selected outcomes were reported |
All selected outcomes were reported |
| Group imbalance | A | High risk | High risk |
| - | B | Implants of different types were used | Implants of different types were used |
| Sample size | A | High risk | High risk |
| - | B | No sample size calculation was performed | No sample size calculation was performed |
| Follow up time | A | Low risk | Low risk |
| - | B | Patients were recalled every 6 months for 1 year |
The mean duration of follow up was 36-98 months. |
| Clinician bias | A | Low risk | Low risk |
| - | B | The study addressed and specified each of the 3 surgeon performed the interventions; the same for the prosthetic treatment |
One surgeon performed all the interventions |
| Radiographic outcome | A | Low risk | Low risk |
| - | B | The independent investigators conducted the radiographic measurements | An independent examiner interpreted all radiographs |
| Authors, Years/ Refs. | Representativeness of the Exposed Cohort |
Selection of External Control |
Ascertainment of Exposure |
Outcome of Interest not Present at Start |
Comparability of Cohorts on the Basis of the Design or Analysis |
Assessment of Outcome |
Follow-up Period Long Enough for Outcomes to Assess |
Adequacy of Follow-up of Cohorts |
Total |
|---|---|---|---|---|---|---|---|---|---|
| Manso et al, 2010 [33] |
* | * | * | * | * | * | * | * | 8 |
| Simonpieri et al, 2011 [35] |
0 | * | * | * | * 0 |
* | * | * | 7 |
| Canullo et al, 2012 [34] |
* | 0 | * | * | * 0 |
* | * | * | 7 |
| Rodriguez et al, 2003 [32] |
* | * | * | * | 0 | * | * | * | 7 |
3. RESULTS
The initial search found 1216 articles. Following the removal of duplicate and irrelevant studies that failed to meet the pre-defined inclusion criteria, this review proceeded with an analysis of nine articles. The process of identifying these nine articles is illustrated in Fig. (1). Of the 9 articles reviewed, one was a randomized controlled clinical trial [28], two were controlled clinical trials [11, 29], one was a case report [30], and five were prospective cohort studies [31-35].
Demographic data from the nine included articles indicated a total of 416 participants. A total of 801 implants were placed in the atrophic posterior maxillae of 416 patients. All studies investigated simultaneous implant placement during lateral sinus floor augmentation in cases with less than 5 mm of residual bone height.
3.1. Evolution of Maxillary Sinus Floor Augmentation
Maxillary sinus floor augmentation (MSFA), also known as sinus floor elevation, has become a standard procedure for addressing bone loss in the posterior maxilla. This technique allows for the placement of dental implants in areas where bone has been compromised due to sinus pneumatization, alveolar bone atrophy, or trauma. Hilt Tatum's pioneering work in the 1970s [12] established the concept of using the maxillary sinus cavity to increase bone volume with graft materials, leading to greater implant-to-bone contact once the graft matures. While the initial procedure described by Boyne and James [5] differed from current practices, it laid the foundation for modern MSFA techniques. Since then, a vast body of research has emerged exploring various grafting materials and modifications to the procedure [36, 37].
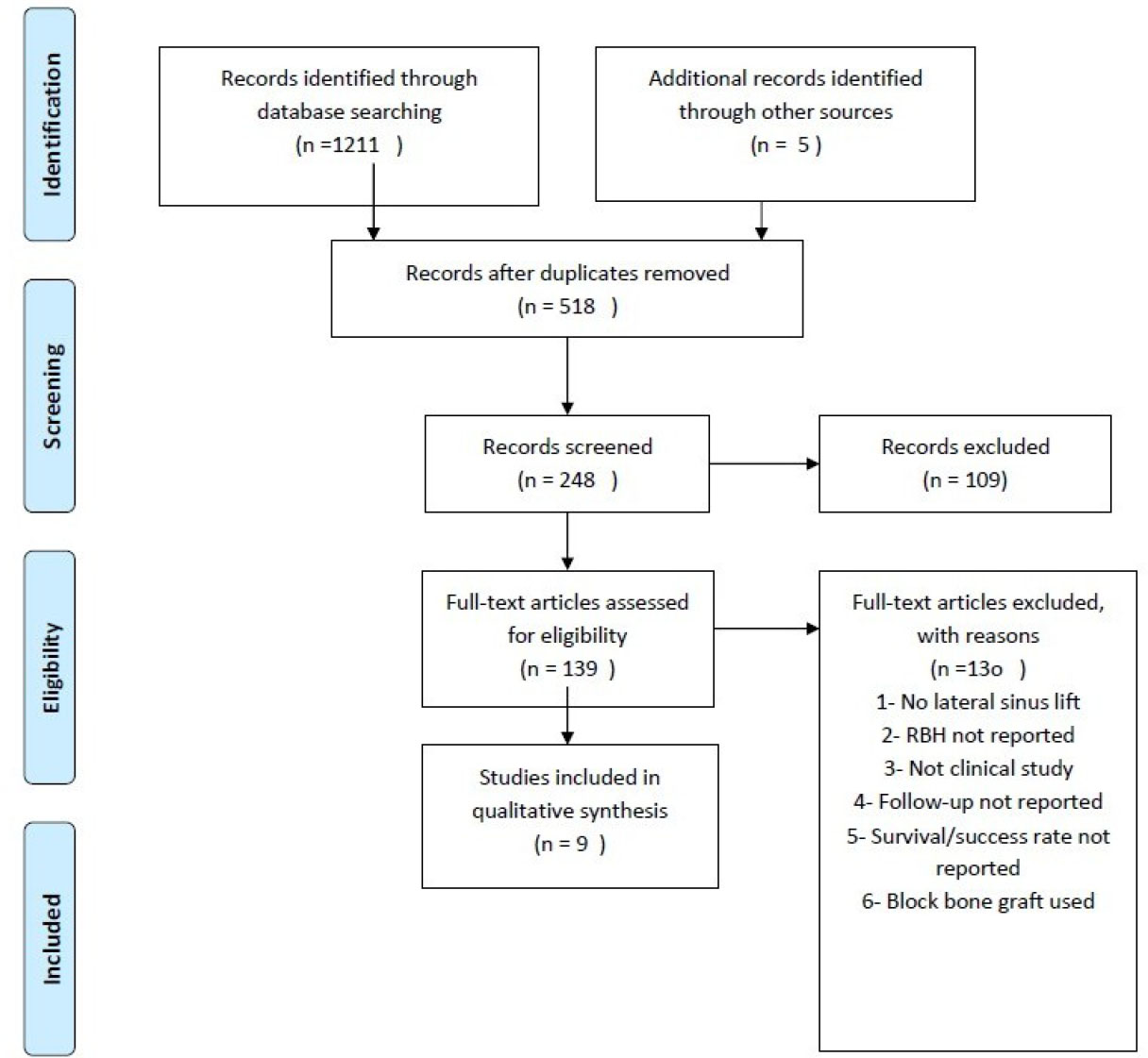
The PRISMA flow diagram of the selection process.
The maxillary sinus, which is the largest paranasal sinus, is a pyramid-shaped cavity with average dimensions of 36-45 mm in height, 23-25 mm in width, and 38-45 mm in length. Its volume averages 15 ml [38].
The sinus's anterior wall extends from the inferior orbital rim to the maxillary alveolar process, containing the infraorbital neurovascular bundle. The thin superior wall forms the floor of the orbit. The posterior wall separates the sinus from the pterygopalatine fossa, which houses the posterior superior alveolar nerve and blood vessels, the pterygoid plexus of veins, and the internal maxillary artery.
The medial wall, the lateral wall of the nasal cavity, contains the primary ostium, the main drainage channel for sinus secretions. The lateral wall, forming the buccal aspect of the sinus, contributes to the posterior maxillary and zygomatic processes and provides access for lateral wall sinus graft procedures.
Maxillary sinus septa, first described by Underwood in 1910 [39], can be classified as primary (formed during maxillary and teeth development) or secondary (acquired after tooth loss) [40].
Most septa are located between the second premolar and first molar [41] and can complicate sinus augmentation procedures. If a septum fully divides the sinus, multiple lateral windows are created during sinus opening to bypass the septum [42].
The maxillary sinus is lined by the Schneiderian membrane, a pseudostratified columnar respiratory epithelium with cilia. This membrane, typically 0.13-0.5 mm thick, is composed of basal, columnar, and goblet cells [43].
It must be fully detached from the sinus floor for successful elevation. However, the distal portion of the sinus can extend significantly [44].
Sinus membrane perforation risk is related to the angle between the lateral and medial walls. Angles greater than 60° have no perforation risk, while angles between 30° and 60° have a 28.6% risk, and angles less than 30° have a 62.5% risk [45].
Overfilling the sinus with graft material can lead to membrane necrosis, sinusitis, and graft loss.
The maxillary premolars and molars have a close relationship with the sinus, with molar roots being closer than premolar roots [46].
The mesiobuccal root apex of the second molar is closest to the sinus wall (average 0.83 mm), while the lingual root apex of the first premolar is furthest [47].
The maxillary sinus receives blood supply from branches of the maxillary artery, including the infraorbital, posterior lateral nasal, and posterior superior alveolar arteries. The greater palatine artery may also contribute to the inferior portion [48].
The lateral wall is supplied by the infraorbital and posterior superior alveolar arteries, while the medial wall receives blood from the posterior lateral nasal artery.
The lateral wall features both extraosseous (buccal tissues) and intraosseous (buccal bone plate) anastomoses between the infraorbital and posterior superior alveolar arteries. The extraosseous anastomosis, located around 23–26 mm from the ridge, can cause bleeding during flap preparation. The intraosseous anastomosis, approximately 16–19 mm from the ridge, may appear as a radiolucency on CBCT scans. This intraosseous vessel must be considered during lateral window preparation to avoid excessive bleeding [49].
The selection of a maxillary sinus elevation and augmentation technique is influenced by both the surgeon's preference and the patient's individual anatomy. Factors such as the remaining bone height and the desired amount of lift play a significant role in this decision. Thus, the decision of selecting a direct approach is related to lower alveolar bone height.
Two primary approaches exist: Direct and Indirect [50].
The direct approach utilizes a lateral window technique. Indirect approaches include:
1- Osteotome sinus floor elevation
2- Bone-added sinus floor elevation
3- Minimally invasive transalveolar sinus approach
4- Antral membrane balloon elevation
The direct/lateral window technique involves direct visualization and instrumentation of the sinus membrane through an opening in the maxillary sinus's lateral wall.
3.2. Steps of the Technique
3.2.1. Anesthesia
Starting with infraorbital, posterior superior alveolar, and greater palatine nerve blocks, along with subperiosteal anesthesia via slow infiltration (1 ml/min).
3.2.2. Incision
By creating a soft-tissue incision at least 10-15 mm anterior to the sinus wall, followed by a mid-crestal incision using a 15C blade. Then, raising a full-thickness flap to access the canine fossa, zygomatic arch, and posterior maxillary wall while ensuring the periosteum remains intact.
3.2.3. Lateral Window/Antrostomy
A specific outline of the window on the buccal bone is planned based on the sinus and implant requirements (typically 20 mm mesiodistally and 15 mm apicocoronally). A high-speed handpiece is used to create the window, avoiding sharp edges. Depending on access, the antrostomy may be elevated or removed entirely.
3.2.4. Sinus Membrane Elevation
By carefully detaching and elevating the sinus membrane using blunt instruments and curettes, ensuring integrity by monitoring the membrane during patient breathing.
3.2.5. Implant Site Preparation
If 3-4 mm of quality residual bone is present, implants can be placed immediately; otherwise, delayed placement of implants 4-6 months after MSFA. Undersized osteotomy is used to protect the sinus membrane.
3.2.6. Graft Placement
The sinus membrane is protected with a collagen membrane, and then the graft is filled in the least accessible areas first, ensuring not to compact tightly to allow for vascularization. Platelet-rich fibrin may also be used as a grafting material.
3.3. Quality Assessment and Risk of Bias of the Included Studies
Assessment of the risk of bias is presented in Tables 2 and 3. The included randomized controlled trials (RCT) and controlled clinical trials (CCT) were well conducted regarding data collection and outcome reporting, indicating a low risk of bias. However, these two studies showed a high risk of bias in participant and surgeon blinding (performance bias), group imbalance, and sample size calculation. In contrast, none of the included prospective cohort studies achieved the highest score on the Newcastle-Ottawa Scale (NOS); however, all studies scored 7 or 8 points, revealing an overall low risk of bias.
4. DISCUSSION
The purpose of this systematic review was to evaluate the survival and success rates of one-stage implant placement in grafted maxillary sinuses using a lateral approach where the initial residual bone height was less than 5 mm.
4.1. The Efficacy of Different Surgical Approaches (two-stage vs. one-stage)
Many studies have evaluated the results of two-stage surgical approaches to restore lost teeth in the posterior atrophic maxilla, achieving good results despite the time required for bone graft maturation. A retrospective study by Friberg et al. (2016) [51] evaluated the clinical and radiographic outcomes of a two-stage surgical technique involving lateral sinus floor elevation using bovine bone (BioOss®) and subsequent implant placement. The study concluded that the implant survival rate was 99.0% considering those engaging BioOss®.
Some attempts to shorten treatment time evaluated both immediate and delayed implant placement after maxillary sinus floor elevation. A retrospective study by Jurisic et al. (2008) [52] investigated the clinical outcomes of dental implants placed in augmented maxillary sinuses using different surgical techniques. The study compared the use of an osteotome versus a lateral approach, along with synchronous or delayed implant placement. The researchers concluded that all groups achieved optimal implant survival rates, with no statistically significant variations observed.
Vertical bone height was less of an obstacle after simultaneous implant placement in severely resorbed alveolar bone. A retrospective clinical study conducted by Pistilli et al. in 2022 [31] examined implant-supported restorations in severely resorbed maxillae (less than 3 mm) following sinus lift procedures using xenografts and guided surgery. The findings indicated that there was no loss of implants after a mean follow-up period of 5.11 years (SD: 2.47). Additionally, no cases of peri-implant mucositis or peri-implantitis were reported throughout the follow-up period. Therefore, simultaneous implant placement alongside lateral sinus floor augmentation in atrophic posterior maxillae did not exacerbate the final outcomes.
Felice et al. (2013) [28] conducted a randomized controlled trial to evaluate the effectiveness of one-stage versus two-stage lateral sinus lift procedures in patients with limited bone height (1-3 mm) and sufficient width (at least 5 mm). The study, which delayed implant placement by four months in the two-stage group, found no signficant difference in implant survival between the two approaches. However, the one-stage procedure was associated with a slightly increased risk of implant failure.
4.2. Implant Survival Rate
Albrektsson and colleagues' success criteria [53, 54] focused on key parameters like marginal bone loss, implant mobility, peri-implant infection with pus formation, and persistent pain. Across the included studies, the implant survival rate ranged from 90% to 100% after an average follow-up of 36 months.
Implant failure rates were further analyzed based on the surgical procedure (one-stage or two-stage). While one-stage techniques exhibited a slightly higher failure rate, the difference was not statistically significant. Multiple clinical studies have supported these findings [32, 34], suggesting that immediate implant placement, when sufficient bone height allows for primary stability, remains a viable and successful surgical approach.
When assessing the factors that influence the survival or success rate of implants [55], it is important to note that only four studies [11, 33, 34, 56] provided data on insertion torque and the crown/implant ratio as potential risk factors for failure. Notably, one study [28] found that in the two-stage procedure group, a significantly higher percentage of implants were inserted with a torque exceeding 30 Ncm (97.9% compared to 18.2%). Additionally, more sites were identified as having soft bone quality for stage-1 implants (81% versus 0%). In contrast, a controlled clinical trial by Cha et al. [11] indicated that if an initial stability of 15 Ncm was not achieved according to the torque gauge, a larger diameter implant was utilized. No other studies provided information on insertion torque.
4.3. Biological Complications
Biological complications during sinus lift procedures were categorized as either intrasurgical (membrane perforation) or post-surgical (acute sinusitis). While membrane perforation was the most frequently reported intraoperative complication, none of the reviewed studies found a link between this complication and implant treatment outcome.
One study [9] examined 217 sinus membranes, finding that 35 were perforated. Despite placing 68 implants in these perforated sites, only 3 implants failed. Statistical analysis (chi-square test with Fisher's exact test) revealed no significant difference in success rates between implants placed in perforated and non-perforated sinuses (p=0.7162). All perforated membranes were sealed with a collagen membrane.
In a study by Simonpieri et al. [35], significant Schneiderian membrane perforations were observed and treated by covering the perforations with PRF membranes. This approach resulted in zero implant loss during the six-year follow-up period.
Post-surgical complications were less common. One study [28] reported a single case of graft infection linked to membrane perforation. Another study [57] described five cases of sensory disturbances due to incisive nerve injury during graft harvesting. Finally, one study [29] reported a case of sinusitis.
Healing abutments are vital components in the healing process that promote the immune responses of bone and mucosa around the implant and improve aesthetic outcomes [58].
Recently, PEEK healing abutments have been widely used due to their physical properties for biomedical applications [59].
A study conducted by Suphangul et al. compared plaque accumulation between PEEK and Ti healing abutments. The study revealed that biofilm formation on the PEEK surface was slightly higher than on the Ti surface; however, no statistical difference (p > 0.05) was found, suggesting that both PEEK and Ti healing abutments can be used as biomaterials for healing abutments according to the requirements of the prostheses in implant dentistry [60].
4.4. Bone Augmentation Biomaterials
Successful bone regeneration, crucial for treating diverse bodily defects, relies heavily on interdisciplinary collaboration between clinicians, bioengineers, and materials scientists. Advances in biomaterials and bone augmentation techniques are rapidly improving outcomes, particularly those involving biodegradable scaffolds [61].
Five categories of bone substitutes exist, based on their chronological development: xenografts, allografts, and autografts; allogeneic bone; natural bone matrices incorporating growth factors; tissue-engineered constructs; and gene-activated bone grafts [62].
Significant advancements have been made in the field of biomaterials and bone augmentation, offering improved methods for implant placement. The incorporation of diverse scaffold materials, stem cells, and growth factors into regenerative treatment strategies shows considerable promise for the repair of maxillofacial defects [63].
A significant deficiency exists in the literature regarding controlled clinical trials evaluating the efficacy of one-stage surgical approaches in cases of critically atrophic maxillae. Therefore, the conduct of such trials is strongly recommended. These trials should incorporate a comprehensive data collection strategy, encompassing measurements of initial bone height, the volume of sinus augmentation performed, the occurrence of any surgical complications, the assessment of implant success according to both clinical and radiographic criteria, long-term follow-up data extending to the prosthetic loading phase, and the utilization of split-mouth study designs.
CONCLUSION
Several existing studies on implant placement in the posterior atrophic maxilla have been presented. While acknowledging limitations in the quality of the available clinical evidence, this review concludes that one-stage and two-stage lateral sinus lift procedures demonstrate comparable implant survival rates. This finding remains consistent across various graft materials. However, a significant challenge in interpreting the data arises from the inconsistent reporting of success and survival rate criteria across studies; several studies failed to explicitly define the criteria used to assess implant survival. This lack of standardized methodology introduces heterogeneity in the results. Despite these limitations, the available data suggest that both one-stage and two-stage approaches, utilizing a range of graft materials, achieve equivalent rates of successful implant integration within the challenging anatomical constraints of the posterior atrophic maxilla. Further research employing robust methodologies and standardized outcome measures is warranted to definitively assess the relative merits of these surgical techniques.
AUTHORS’ CONTRIBUTIONS
The authors confirm their contribution to the paper as follows: N.S.: Writing - Reviewing and Editing; A.K.: Investigation. All authors reviewed the results and approved the final version of the manuscript.
LIST OF ABBREVIATIONS
| GBR | = Guided bone regeneration |
| MSFA | = Maxillary sinus floor augmentation |
| SFE | = Sinus floor elevation |
| RBH | = Residual bone height |
| RCTs | = Randomized controlled trials |
| CCT | = Controlled clinical trials |
AVAILABILITY OF DATA AND MATERIALS
The data that support the findings of this study are available from the corresponding author, [N.S], on special request.
ACKNOWLEDGEMENTS
Declared none.


